Resective Epilepsy Surgery after Corpus Callosotomy in Children with Lennox-Gastaut Syndrome
Article information
Abstract
Purpose
This study examined the characteristics and outcomes of resective epilepsy surgery following corpus callosotomy (CC) in children with Lennox-Gastaut syndrome (LGS).
Methods
We retrospectively analyzed 17 children with LGS who underwent resective surgery (RS) after CC over a span of 10 years, with a minimum of 2 years of follow-up, at a single tertiary epilepsy center in Korea.
Results
Of the 17 patients, 13 (73.5%) demonstrated favorable surgical outcomes (Engel class I or II) at 1 year after RS, and eight (47.1%) were ultimately free of seizures 2 years after surgery. A significantly larger decrease in the number of anti-seizure medications taken from before to 2 years after the final surgical procedure was observed in the group that became seizure-free than in the group with persistent seizures (P=0.062). Furthermore, a significantly greater decline in daily adaptive function was found in the persistent seizure group (P=0.059). The baseline characteristics, results of presurgical evaluation, and treatment-related factors assessed prior to surgery showed no significant differences between the seizure-free group and the group with persistent seizures.
Conclusion
In conclusion, RS may be a viable option for patients with LGS who exhibit lateralization and/or localization on presurgical evaluation after CC, as the procedure may reveal a concealed primary focus. The proactive implementation of two-stage epilepsy surgery could provide significant seizure reduction and preservation of cognitive function in carefully selected patients with LGS.
Introduction
Lennox-Gastaut syndrome (LGS) is a severe form of epileptic encephalopathy, characterized by multiple seizure types that typically begin during childhood [1,2]. Most patients with LGS also experience cognitive and behavioral issues, which are influenced by the frequency and severity of the seizures as well as the treatment methods employed [3-5]. Various treatment strategies have been employed for seizure control in patients with medically intractable LGS. When non-surgical treatments such as anti-seizure medications (ASMs) and the ketogenic diet (KD) fail, a surgical approach is considered. In some patients with LGS, the epileptogenic focus can be identified through extensive presurgical evaluation. This assessment combines brain magnetic resonance imaging (MRI), long-term video electroencephalography (EEG) monitoring, 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (2-FDG-PET), and single-photon emission computed tomography (SPECT) [6-8]. Resection of the focal epileptogenic focus is viewed as a curative surgical method that can yield promising seizure outcomes in patients with LGS [8,9]. While LGS is considered a diffuse secondary generalized encephalopathy, this should not preclude patients from undergoing resective epilepsy surgery. For patients with LGS whose epileptic foci are not easily localized, less invasive palliative surgical options such as corpus callosotomy (CC) and vagus nerve stimulation are also considered [6,7,10]. Interestingly, in a small number of patients who underwent CC due to inconsistent findings regarding clinical factors and the results of various preoperative examinations, successful surgical outcomes have been achieved through subsequent resective surgery (RS). This is thought to be possible due to the revealed hidden focus on EEG findings and seizure type after CC. However, recent studies on the effects of CC with subsequent RS among children with LGS are scarce [11-13]. The aim of this study was to investigate the characteristics and outcomes of RS following CC in children with LGS.
Materials and Methods
1. Patients
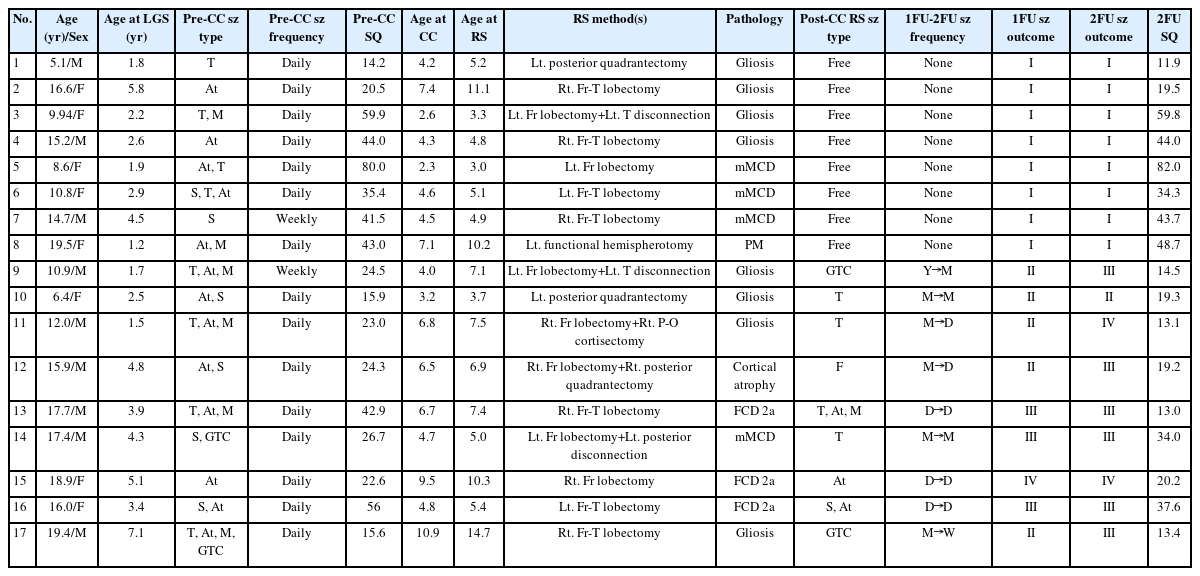
We retrospectively reviewed the medical records of patients with LGS who underwent epilepsy surgery at Severance Children’s Hospital between 2010 and 2020. The patients included in this review met the following criteria: (1) they had multiple types of seizures with generalized slow spike-and-wave (GSSW) discharges and/or generalized paroxysmal fast activity (GPFA) as evidenced on EEG; (2) they exhibited developmental delay or cognitive decline following seizure onset; (3) they underwent a comprehensive pre-surgery evaluation using multiple modalities, including long-term video-EEG monitoring, brain MRI, FDG-PET, SPECT, and a neuropsychological (NP) test; (4) they had a history of two-stage epilepsy surgery, specifically CC followed by RS; and (5) they had a minimum follow-up period of 2 years after the post-CC RS procedure. We excluded patients based on the following criteria: (1) they had confirmed genetic or metabolic causes other than LGS; (2) they had undergone more than three surgical procedures for the treatment of epilepsy; or (3) they demonstrated non-compliance with treatment or evaluation before or after surgery. Among the 173 patients with LGS who underwent epilepsy surgery during this 10-year period, 17 were ultimately included in the review due to fully meeting the above-mentioned inclusion and exclusion criteria.
The need for informed consent was waived by the Severance Hospital Yonsei University and approved by the Institutional Review Board of Severance Children’s Hospital due to the study being retrospective in nature (IRB no. 4-2022-1163).
2. Seizure classification
All children selected for this study had been treated for various types of seizure attacks, having been administered more than three ASMs with limited success. They opted for epilepsy surgery due to (1) debilitating seizures such as generalized tonic seizures, traumatic falling attacks, or head nodding seizures with occasional falling; (2) the burden of multiple ASMs, which often have troublesome side effects; and/or (3) progressive regression caused by unmanageable seizures and treatment over time. The types of seizures experienced by the patients were determined based on reports from caregivers and documentation from video-EEG monitoring. These were then analyzed and categorized according to the 2017 International League Against Epilepsy classification system [14]. Tonic seizures, a common type of seizure in patients with LGS, often occur during sleep. These seizures are typically brief, lasting less than 10 seconds, and can occur in series. Atonic seizures are also frequently observed in these patients. While atonic seizures are typically brief, they often result in traumatic falls. Generalized tonic-clonic seizures, focal seizures, and epileptic spasms are also observed in patients with LGS [3,4]. The frequency of primary habitual seizures was recorded and reported by caregivers. In terms of seizure frequency, we defined a daily seizure as more than a single seizure attack every day, a weekly seizure as less than daily but at least once a week, a monthly seizure as less than weekly but at least once a month, and a yearly seizure as less than monthly but at least once a year.
3. Presurgical evaluation
The presurgical evaluation included long-term video-EEG monitoring, brain MRI, interictal SPECT, FDG-PET, and an NP test. Scalp long-term video-EEG, with electrode placement adhering to the global 10–20 system, was captured using a 19-channel recorder for at least 24 hours. The brain MRI scans included 3-mm-thick axial T1- and T2-weighted, sagittal T1-weighted, coronal T2-weighted, and axial and coronal fluid-attenuated inversion recovery images. MRI findings were classified as either abnormal focal, abnormal multifocal/diffuse abnormal, or normal. Focal abnormalities included malformations of cortical development (MCDs) such as focal cortical dysplasia (FCD), polymicrogyria, pachygyria, or heterotopia and destructive lesions like focal encephalomalacia resulting from previous insult, hemorrhage, or inflammatory change in unilateral focal areas. The abnormal multifocal/diffuse category encompassed MCD, destructive lesions affecting more than two lobes in the unilateral or bilateral hemisphere, and diffuse cortical atrophy. For long-term EEG monitoring, technetium-99m ethyl cysteinate dimer was injected for interictal SPECT, ensuring confirmation of the non-ictal state. FDG-PET was conducted concurrently with EEG. All 17 patients underwent a comprehensive NP test prior to surgery.
4. Surgical process and pathologic findings
All patients initially underwent CC due to the absence of definitive evidence of a primary seizure focus on the presurgical evaluations described earlier. In other words, the decision to first perform CC was made because the patients either exhibited normal findings on brain MRI or displayed discordant findings on EEG, PET, and SPECT, despite the presence of suspicious lesions on brain MRI. Following CC, most patients experienced an initial decrease in both the frequency and intensity of their seizures. However, after several months, the seizures worsened, and/or EEG showed signs of lateralization after CC. Lateralization or localization on EEG is characterized by unilateral sharp wave discharges with a decrease in or even resolution of GSSW discharges/GPFAs, or dominant focal sharp wave discharges or spikes observed on EEG. This indicates a primary seizure as a pathological lesion. These patients were then re-evaluated using all of the presurgical tests previously mentioned. Patients whose long-term video-EEG results consistently suggested a possible primary focus and/or correlated with PET and SPECT results underwent RS as a second surgical procedure. The types of RS used for epilepsy include cortisectomy, single lobectomy, multilobar lobectomy (sometimes combined with disconnection), and functional hemispherotomy. Histopathologic results such as mild MCD, FCD, cortical atrophy, and gliosis were classified according to the International League Against Epilepsy and Palmini classifications [15].
5. Seizure and developmental outcomes
The seizure outcome was evaluated based on the Engel Epilepsy Surgery Outcome Scale, as follows: class I represents freedom from disabling seizures, class II indicates rare disabling seizures (also referred to as “almost seizure-free”), class III signifies a worthwhile improvement, and class IV denotes no worthwhile improvement [8]. The seizure-free group consisted of eight patients who remained seizure-free for 2 years following RS. The persistent seizure group included those patients whose seizures continued even 2 years post-surgery.
Developmental outcomes were assessed using the social quotient (SQ). Many patients with moderate to severe intellectual disabilities were unable to fully participate in the Wechsler Preschool and Primary Scale of Intelligence test, leading to invalid results. As formal intelligence quotient tests were not feasible for most patients, the study maintained consistency in the developmental evaluation scale by substituting SQ for intelligence quotient. The SQ was determined using the Korean Social Maturity Scale, a tool designed to evaluate individuals aged 0 to 30 years on their personal and social skills pertinent to daily living. The median values for SQ were compared from before to 2 years after RS. The statistical significance of the difference between the two groups was then assessed.
6. Statistical analyses
Demographic and clinical characteristics are presented as median values (interquartile range [IQR]) for continuous variables and as frequencies (percentages, %) for categorical variables. The Mann-Whitney U test and the Fisher exact test were employed to compare the clinical characteristics between the seizure-free and persistent seizure groups (the Mann-Whitney U test for median comparisons and the Fisher exact test for group comparisons of categorical variables). All analyses were conducted using a two-tailed approach. P values of less than 0.1 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Results
1. Patients
The median age at onset of LGS in the 17 patients (nine boys and eight girls) was 2.9 years (IQR, 1.8 to 4.95). The patients underwent CC at a median age of 4.67 years (IQR, 4.13 to 6.94), and the latent period from LGS onset to CC was 1.7 years (IQR, 0.57 to 3.29). Eleven (64.7%) patients were treated for West syndrome prior to the onset of LGS. Due to the medically intractable nature of their seizures, all patients had tried more than three ASMs (median, 5 [IQR, 4 to 5]), and 14 (82.3%) patients had a history of using KD for seizure control before undergoing CC. Most of the patients exhibited moderate to severe preoperative adaptive functions, as shown in Table 1.
2. Seizure classification
The most common seizure type was atonic seizure (n=13, 76.5%), which can be associated with traumatic falling. Brief tonic seizures were also a primary habitual seizure type (n=8, 47.1%). Most patients (n=15, 88.2%) experienced these primary seizures daily, despite numerous adjustments to their ASMs (Table 1). Patient 7 experienced clustered spasms, which were relatively well-controlled with only two ASMs, occurring on a weekly basis. However, his brain MRI and EEG indicated focal lesions even prior to CC. Initially, he underwent CC due to inconsistent findings from PET and SPECT scans, but he was later identified as an RS candidate due to strong evidence of localization on his post-CC EEG. Patient 9 also experienced weekly seizures, but the disabling nature of his seizure types and developmental regression, despite multiple ASMs, ultimately necessitated surgery (Table 2).
3. Presurgical evaluation
Among the 17 patients studied, brain MRI revealed focal lesions in two (11.8%), multifocal/diffuse lesions in seven, and normal results in eight (47.1%) despite the use of 3-mm-thick high-resolution MRI. These results were meticulously reviewed by expert radiologists and pediatric neurologists (Table 3). Prior to CC, long-term video-EEG monitoring revealed active GSSW discharges and/or GPFA in all 17 patients. Multifocal spikes in more than three areas over bilateral hemispheres were observed in 16 (93.1%) patients, while six (35.3%) patients exhibited unilateral dominant spikes/sharp wave discharges. EEG lateralization was defined as indicating more than 70% dominance in one hemisphere or a single focus. FDG-PET and interictal SPECT results were partially concordant in three (17.6%) and six (35.3%) patients, respectively. However, these results did not provide sufficient evidence to proceed with resection surgery. Due to the discordant presurgical evaluation results, CC was performed for these 17 patients (Table 3). At least 3 months after CC, EEG evaluations of all 17 patients showed a tendency towards lateralization, indicating dominant focal sharp/slowing or localized paroxysmal fast activities. This suggested the presence of a primary focus. Following CC, the resolution of GSSW discharges and/or GPFA was observed in 11 (65.7%) patients, while eight (47.1%) patients continued to exhibit multifocal spike activities. In addition to these EEG results, correlations between FDG-PET and interictal SPECT significantly increased post-CC. Fourteen (82.4%) patients showed concordant regions of glucose hypometabolism on FDG-PET, and 13 (76.5%) patients demonstrated a correlation between interictal SPECT and the localized or lateralized EEG findings (Table 3).
4. Surgical process and pathologic findings
Seven patients (41.2%) underwent multilobar resection, while five patients (29.4%) were treated with combined surgery. The latter included focal lobectomy and cortisectomy, temporal lobe disconnection, or posterior quadrantectomy. Two patients (11.8%) underwent frontal lobectomy, and another two patients (11.8%) underwent posterior quadrantectomy. Functional hemispherotomy was performed in only one patient (5.9%) (Table 2). The median age at which these patients underwent RS was 5.38 years (IQR, 4.84 to 8.88), and the latent period from the onset of LGS to RS was 2.24 years (1.19 to 5.41). Nonspecific gliosis and MCD were the most common pathological findings, each observed in eight patients (47.1%). Mild MCD was found in four patients (23.5%), FCD type IIa was observed in three patients (17.6%), and polymicrogyria was noted in the remaining patient with MCD (5.9%). Cortical atrophy was observed in one patient (5.9%), whose brain MRI was normal (Table 2).
5. Seizure and developmental outcomes
Prior to CC, 15 of the 17 patients (88.2%) experienced daily intractable seizures. These included brief tonic, myoclonic, atonic, and generalized tonic-clonic seizures, as well as occasional clustered spasms (Tables 1 and 2). Two years after CC and subsequent RS, eight patients (47.1%) were seizure-free, and 10 of the 17 (58.8%) patients exhibited favorable seizure outcomes, falling into Engel class I or II (Table 2). From before to 2 years after the final surgical procedure, a significantly greater decrease in daily adaptive function was found in the persistent seizure group compared to the seizure-free group (P=0.059) (Fig. 1A). Furthermore, a significantly larger decline in the number of ASMs taken was observed in the seizure-free patients than in those with persistent seizures (P=0.062) (Fig. 1B). The baseline characteristics, results of presurgical evaluation, and treatment-related factors assessed prior to surgery showed no significant differences between the seizure-free and persistent seizure groups (Table 4).
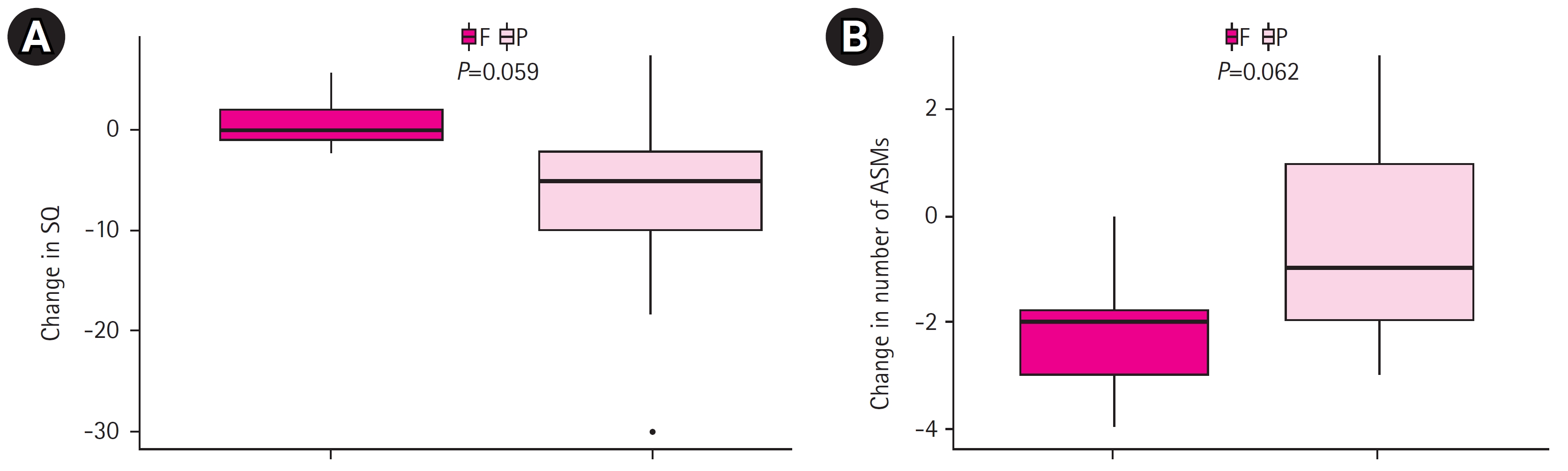
(A) Changes in adaptive function, as measured by social quotient (SQ), in patients with Lennox-Gastaut syndrome (LGS) from prior to corpus callosotomy (CC) to 2 years after subsequent resective surgery (RS). (B) Changes in the number of anti-seizure medications (ASMs) taken by patients with LGS from prior to CC to 2 years after subsequent RS. F, seizure-free group; P, persistent seizure group.
Discussion
Our analysis indicates that CC plays a crucial role in identifying the primary epileptogenic focus in patients with medically intractable LGS with active generalized bilaterally synchronous spikes. This can ultimately enable RS and yield a significant reduction in seizures. CC is a common procedure in palliative epilepsy surgery for nonresectable cases. However, it disrupts the GSSW pattern in patients with LGS, which results in more pronounced EEG findings with lateralization of the primary focus and the disappearance of spikes in the contralateral hemisphere [12,16,17]. Moreover, CC alters the topology of the functional brain network and the anatomical covariance network [18-20]. Changes in functional neuroimaging modalities, such as FDG-PET or SPECT, following CC are also beneficial in detecting the primary epileptogenic focus. Given these roles and effects, in addition to the original role of callosotomy as palliative surgery, some patients with an unknown primary focus prior to CC can be identified and subsequently undergo successful RS. In cases of LGS, RS can reduce the frequency of seizures in 70% to 80% of patients for which EEG discharges are dominant in one hemisphere [7-9,17].
In our study, identifying the resection focus through presurgical evaluation proved challenging for the 17 patients with LGS who were being prepared for CC. Of these patients, only two (11.8%) exhibited focal abnormalities, while seven (41.2%) displayed multifocal or diffuse abnormalities on brain MRI. However, these findings did not align with those from EEG and functional neuroimaging. The remaining eight patients (47.1%) showed no clear abnormalities on their brain MRIs. As a result, all patients underwent CC as the initial procedure rather than RS. Our center’s approach to this two-stage surgery hinged on the active application of repeated intensive presurgical evaluations. This method was utilized for patients who demonstrated disruption of GSSW and/or lateralization of epileptiform discharges, as well as the disappearance of spikes in the contralateral hemisphere in EEG following CC. This approach contributed to effective seizure control and improved cognitive and functional states. A recent prospective study on combined surgical procedures (RS following CC) reported that 86.9% of children either were seizure-free or rarely experienced disabling seizures at 1 year of follow-up. This percentage was 81.6% at 3-year follow-up and 76.4% after 5 years [11]. Various surgical treatments are widely recognized as beneficial options for reducing seizures [6-8,17,21]. However, given the potential risks and substantial economic burden associated with epilepsy surgery, the need to identify predictive factors of success prior to surgery is gaining importance [21-23]. To explore these predictive clinical factors, we compared the demographic characteristics and clinical factors of the patients who became seizure-free with those of the patients who did not. Despite these efforts, no statistically significant predictive factors were found (Table 4).
Although this study had limitations, such as its retrospective design, selection bias, and brief follow-up period, the patient sample size was notably larger in comparison to previous studies on CC followed by RS [11,12,16]. Future research focusing on preoperative brain connectivity analysis, the use of magnetoencephalography, and more comprehensive EEG analysis (for instance, a detailed examination of the frequency of focal paroxysmal rapid activity, focal slow spikes and waves, and focal sharps/spikes) may enable the prediction of more suitable surgical candidates. This could potentially lead to increased success rates for epilepsy surgery. We look forward to identifying predictive presurgical biomarkers in a follow-up study.
Notes
Hoon-Chul Kang, Joon Soo Lee, and Heung Dong Kim are an editorial board members of the journal, but they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts.
Author contribution
Conceptualization: HDK. Data curation: SP, HEK, CMK. Formal analysis: SP. Funding acquisition: SP. Methodology: YJH, HDK. Visualization: SP. Investigation: SP, HEK, CMK. Writing-original draft: SP. Writing-review & editing: YJH, HCK, JSL, HDK.
Acknowledgements
We thank the patients and their families for participating in this study. This study was supported by the Soonchunhyang University Research Fund 2023.