Characteristics of Epilepsy in Children with Cerebral Palsy: A Single Tertiary Center Study
Article information
Abstract
Purpose
The aim of this study was to describe the characteristics of epilepsy in cerebral palsy (CP) patients and identify risk factors for epilepsy and drug-resistant epilepsy.
Methods
CP patients aged 18 years old or younger who visited the pediatric neurology department and/or rehabilitation department of a tertiary care hospital between January 2016 and December 2022 with a minimum follow-up period of 2 years were included. Demographic and clinical data, seizure characteristics, brain imaging, electroencephalography, and genetic evaluation results were reviewed retrospectively.
Results
Among 268 patients included in this study, 36.9% had epilepsy and 10.8% had drug-resistant epilepsy. Asphyxia (29.3%), hemorrhage, infarction, and brain infection (25.3%) were associated with epilepsy. Epileptic CP patients were more likely to experience neonatal seizures (18.2% vs. 4.1%, P<0.001) and febrile seizures (12.1% vs. 7.1%, P=0.02) than non-epilepsy CP patients. The most common cerebral subtype in patients with epilepsy was spastic quadriplegia (59.6%). Epilepsy patients were more severely impaired in gross motor function, with worse intellectual disability. Patients with macrocephaly or cerebral malformation were more likely to have drug resistance. Valproate (51.7% and 25.7%) and levetiracetam (41.4% and 25.7%) were the two most commonly used antiseizure medications, both in monotherapy and polytherapy.
Conclusion
A history of asphyxia, febrile seizure, neonatal seizure, spastic quadriplegia, more severely impaired gross motor function, and intellectual disability were found to be risk factors for epilepsy. Further research with prospective data collection to develop a model for predicting seizures or epilepsy in CP patients is needed.
Introduction
Cerebral palsy (CP) is an umbrella term for nonprogressive motor disease caused by insults to a developing brain. CP is a group of heterogeneous disorders resulting from various types of brain injuries during prenatal, perinatal, and postnatal periods [1]. The prevalence of CP is 1.5 to 6 per 1,000 live births in high-income countries and higher in developing countries [1,2]. The prevalence in South Korea is 2 to 4 per 1,000 live births [3,4]. Patients with CP have comorbidities such as intellectual disability (ID), epilepsy, visual impairment, hearing impairment, psychiatric disorders, musculoskeletal problems, and nutritional problems, necessitating extensive lifelong care in health, society, and education contexts [1].
Several studies have shown that 15% to 60% of CP patients have epilepsy as a comorbid disease, and a report using the Korean Database of Cerebral Palsy in 2017 stated that one out of four patients had epilepsy [5-10]. The prevalence of epilepsy in the general population is 3 to 8 per 1,000 persons, and the prevalence in patients with CP is higher than that [8,9,11,12]. The aim of this study was to investigate the characteristics of CP patients in Korea and identify risk factors for epilepsy.
Materials and Methods
Medical records of pediatric patients (≤18 years of age) who visited the pediatric neurology and/or rehabilitation Department of Daejeon St. Mary’s Hospital, a university-affiliated tertiary hospital, from January 2016 to December 2022 with magnetic resonance imaging (MRI) and electroencephalography (EEG), who had a minimum follow-up period of 2 years, and who were diagnosed with International Classification of Diseases, 10th Revision codes of G800, G801, G802, G803, G804, G808, and G809 (CP) were retrospectively reviewed.
Epilepsy was diagnosed if two unprovoked seizures with a minimum interval of 24 hours occurred. Neonatal seizures were defined as clinical seizures confirmed with either video EEG, conventional EEG, or amplitude-integrated EEG occurring between birth and 28 days after birth. Febrile seizures were defined as fever-provoked seizures in children between 6 and 60 months old without a previous diagnosis of epilepsy. Drug-resistant epilepsy (DRE) was defined as the presence of uncontrolled seizures despite adequate doses of two appropriately chosen antiseizure medications (ASMs). Patients with epilepsy who were not included in the drug-resistant group were labeled as those with controlled epilepsy.
The subtypes of CP were defined by modifying the hierarchical classification tree of CP subtypes in Surveillance of Cerebral Palsy in Europe (SCPE) by dividing the spastic bilateral group into spastic quadriplegia and spastic diplegia based on whether the upper limbs were affected. Gross motor function was classified into five groups (group 1 with minimal and group 5 with maximal disability) using the Gross Motor Function Classification System-Expanded and Revised (GMFCS-E&R) at a minimum age of 2. ID was defined when the intellectual quotient (IQ) was below 70. It was divided into four groups: profound if below 20, severe if between 20 and 34, moderate if between 35 and 49, and mild if between 50 and 69. Cryptogenic CP was diagnosed for patients without apparent or possible brain insults, such as prematurity, hypoxic ischemic encephalopathy, infarction, encephalitis, head trauma, periventricular leukomalacia, or findings on brain imaging to explain motor dysfunction. CP patients were subdivided according to the presence or absence of epilepsy. Epilepsy patients were grouped into those with DRE and those with controlled epilepsy. In a subgroup analysis based on CP subtypes (spastic quadriplegia, diplegia, and hemiplegia), patients were classified according to the presence or absence of epilepsy.
We collected demographic and clinical data for sex, gestational age, birth weight, mode of delivery, multiple pregnancy, 5-minute Apgar score, maternal factors, intrauterine growth retardation, birth weight for gestational age, brain insult (including hemorrhage, infarction and infection), occipitofrontal circumference, congenital anomaly, admission to the neonatal intensive care unit (NICU), family history of CP, epilepsy, neurologic diseases, subtype of CP, gross motor function, comorbidities, neonatal and febrile seizures, seizure characteristics, ASM, medications related to spasticity and psychiatric diseases, EEG, brain MRI findings, and results of a genetic evaluation.
The Mann-Whitney test was used for comparing continuous variables between two groups. The chi-square test and Fisher’s exact test were used for categorical variables. A subgroup analysis of CP subtypes (spastic quadriplegia, diplegia, and hemiplegia) was done in the same manner. We used SPSS version 29 (IBM Corp., Armonk, NY, USA) to perform all statistical analyses. A P value less than 0.05 was considered to indicate statistical significance. This study was approved by the Institutional Review Board of The Catholic Medical Center with a waiver for the requirement to obtain informed consent (approval number and date: DC22RASI0003, February 15, 2022).
Results
In total, 268 patients were included in this study, and 60.8% were male. Table 1 shows patients with a spastic diplegia pattern accounted for 35%. Those with spastic quadriplegia accounted for 34.3% and 2.2%, respectively. More than half (57.8%) of CP patients had ID, and the majority of them (67.1%) had an IQ of less than 35, corresponding to severe to profound mental retardation. Excluding neonatal seizures and febrile seizures, 42.3% of patients experienced first seizure during infancy. Among CP patients, about one-third (36.9%) had epilepsy and one-third (10.8%) showed multidrug resistance. In addition, 3.4% of patients experienced status epilepticus.
Table 2 compares demographic and clinical features between epilepsy and non-epilepsy groups. Prematurity (i.e., babies born with a gestational age of less than 37 weeks) was significantly more common in patients without epilepsy (65.7%) than in patients with epilepsy (50.5%, P=0.02). Patients with asphyxia (29.3% vs. 13.0%, P=0.001) or brain injury including brain hemorrhage, infarction, and meningoencephalitis (25.3% vs. 13.0%, P=0.013) were twice as common in patients with epilepsy than in patients without epilepsy. Microcephaly, defined as an occipitofrontal circumference below 2 standard deviations from the mean of age and sex, was more prevalent in the epilepsy group than in the non-epilepsy group (58.6% vs. 36.1%, P=0.001). The NICU admission rate and duration of NICU admission did not show statistically significant differences. Spastic quadriplegia (59.6%) was the most common type in the epilepsy group. Spastic diplegia was the most common (46.2%) type in the non-epilepsy group, followed by spastic hemiplegia (23.7%), with statistical significance (P<0.001). The epilepsy group had a significantly higher frequency of gross motor impairment (51.5% in level 5 GMFCS-E&R vs. 11.2%, P<0.001) and ID (85.9% vs. 41.4%, P<0.001). More patients in the epilepsy group experienced neonatal seizures (18.2% vs. 4.1%, P<0.001) and febrile seizures (12.1% vs. 7.1%, P=0.02) than in the non-epilepsy group. The most common brain imaging results in both the epilepsy group (37.4%) and non-epilepsy group (58.0%) were periventricular leukomalacia and/or periventricular white matter injury. Intracranial hemorrhage (14.1% vs. 1.8%), diffuse gray matter injury (13.1% vs. 3.6%), and deep brain gray matter injury (8.1% vs. 0.0%) were more prevalent in the epilepsy group than in the non-epilepsy group. One-fourth of patients were evaluated with genetic tools. Of them, 35.5% had genetic mutations responsible for their symptoms. The diagnostic yield was 14.0% (6/43) for chromosomal microarrays and 44.1% (15/34) for targeted panel sequencing. None of these patients simultaneously had a copy number variation and single-nucleotide polymorphism. Some of the copy number variants identified in this study included 14q12 deletion, 14q32 duplication, and 22q13 deletion. The genes revealed in our patients from targeted panel sequencing were MBD5, SMC1A, NSD1, NFIX, AFF2, TRIO, SLC2A1, CACNA1A, GNAO1, NIBPL, PROC, and PLAA.
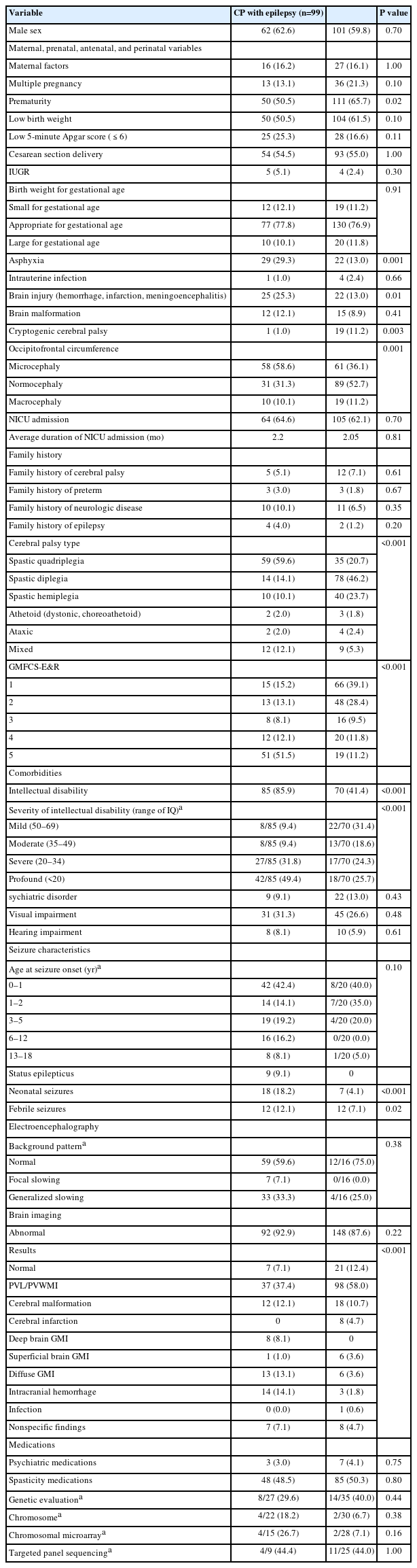
Comparison of demographic and clinical features of cerebral palsy patients with and without epilepsy
We performed a subgroup analysis for three CP subtypes (spastic quadriplegia, diplegia, and hemiplegia), excluding athetoid, ataxic, and mixed CP due to the small sample size. Fig. 1 shows the distribution of variables in the epilepsy and non-epilepsy groups according to CP subtypes. Prematurity, low birth weight, and asphyxia were not significantly more common in the epilepsy group according to CP subtypes. However, a birth history of prematurity, low birth weight, and asphyxia were significantly different between CP subtypes in patients without epilepsy (P=0.009, P=0.003, and P=0.016, respectively). Low birth weight in the epilepsy group (P=0.01) also showed a significant difference between CP subtypes. Comparing the epilepsy and non-epilepsy groups by CP subtypes showed a significantly higher frequency of brain injury in spastic quadriplegic patients (44.1% vs. 20.0%, P=0.03) and spastic hemiplegic patients (20.0% vs. 10.0%, P=0.007). However, there was no statistically significant difference in the presence of brain injury among spastic diplegic patients (7.1% vs. 3.8%, P=0.08). The 5-minute Apgar score, cesarean section delivery, intrauterine growth retardation, intrauterine infection, and brain malformation did not show significant differences in a subgroup analysis by CP subtypes or between CP subtypes in the epilepsy and non-epilepsy groups as shown in Supplementary Fig. 1.
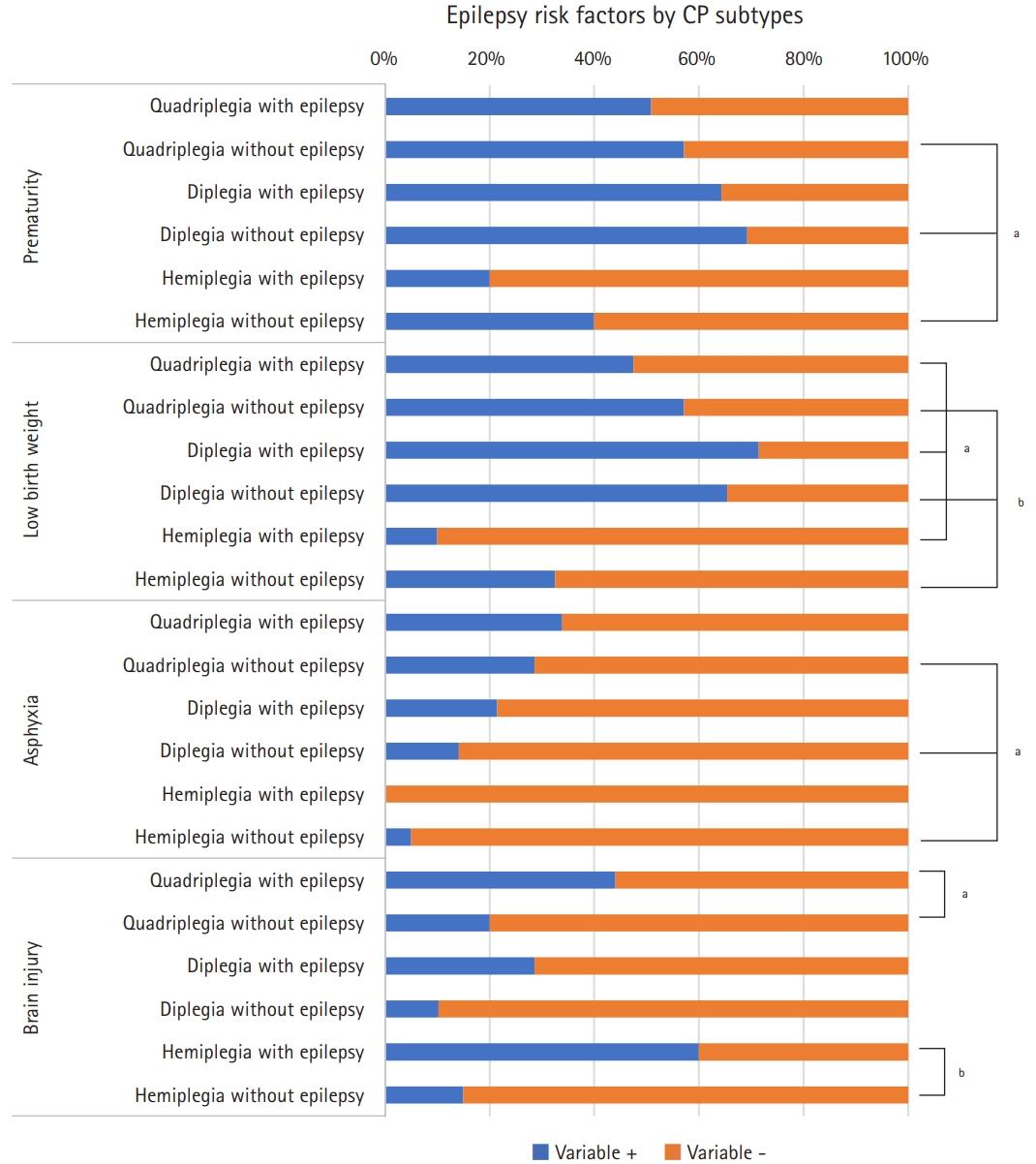
Statistically significant epilepsy risk factors in the subgroup analysis. CP, cerebral palsy. aP<0.05; bP<0.01.
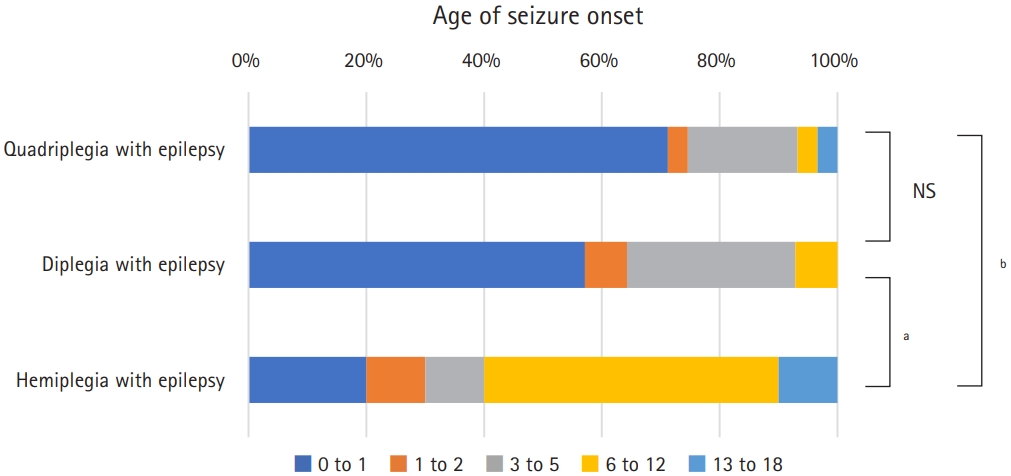
Fig. 2 shows the results of a subgroup analysis comparing epilepsy and non-epilepsy groups by CP subtypes according to the birth weight for gestational age. More patients without epilepsy in the spastic diplegia subgroup had an appropriate birth weight for gestational age defined as 10th to 90th percentile of birth weight to gestational age than those with epilepsy (P=0.03). Fig. 3A shows the proportion of patients with normal intelligence or ID and the grade of ID according to CP subtypes and the presence of epilepsy. For three subtypes of CP, the non-epilepsy group had significantly less ID and milder grades of ID (P<0.001 for spastic quadriplegia and diplegia, P=0.003 for spastic hemiplegia). Of non-epileptic spastic hemiplegia patients, 83.8% had normal intelligence, while only 13.3% had normal intelligence in those with epileptic spastic quadriplegia. The degree of gross motor function in subgroups are described according to the levels of GMFCS-E&R, as shown in Fig. 3B. Patients with mildly impaired gross motor function (GMFCS-E&R levels 1 and 2) were more prevalent in the non-epilepsy groups for all three CP subtypes. The differences were statistically significant in the spastic quadriplegia (P=0.003) and hemiplegia (P=0.01) subgroups, but not in the spastic diplegia subgroup (P=0.63). Fig. 4 shows the distribution of age of seizure onset (excluding neonatal and febrile seizures) according to CP types. The age of seizure onset did not show a statistically significant difference (P=0.51) between spastic quadriplegia and diplegia groups. Those with seizure onset before age 1 accounted for 71.4% of the spastic quadriplegia group and 58.3% of the spastic diplegia group. Spastic hemiplegia had a statistically significant difference in the age distribution of seizure onset compared to the quadriplegia (P<0.001) and diplegia (P=0.046) groups. In total, 22.2% of spastic hemiplegic patients with epilepsy had seizure onset before age 1.
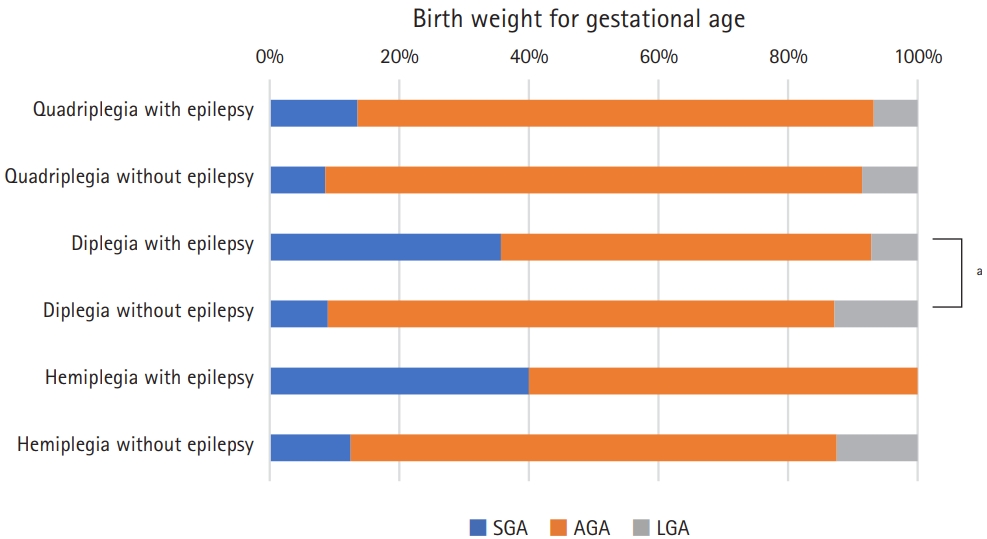
Birth weight for gestational age according to cerebral palsy subtypes. SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age. aP<0.05.
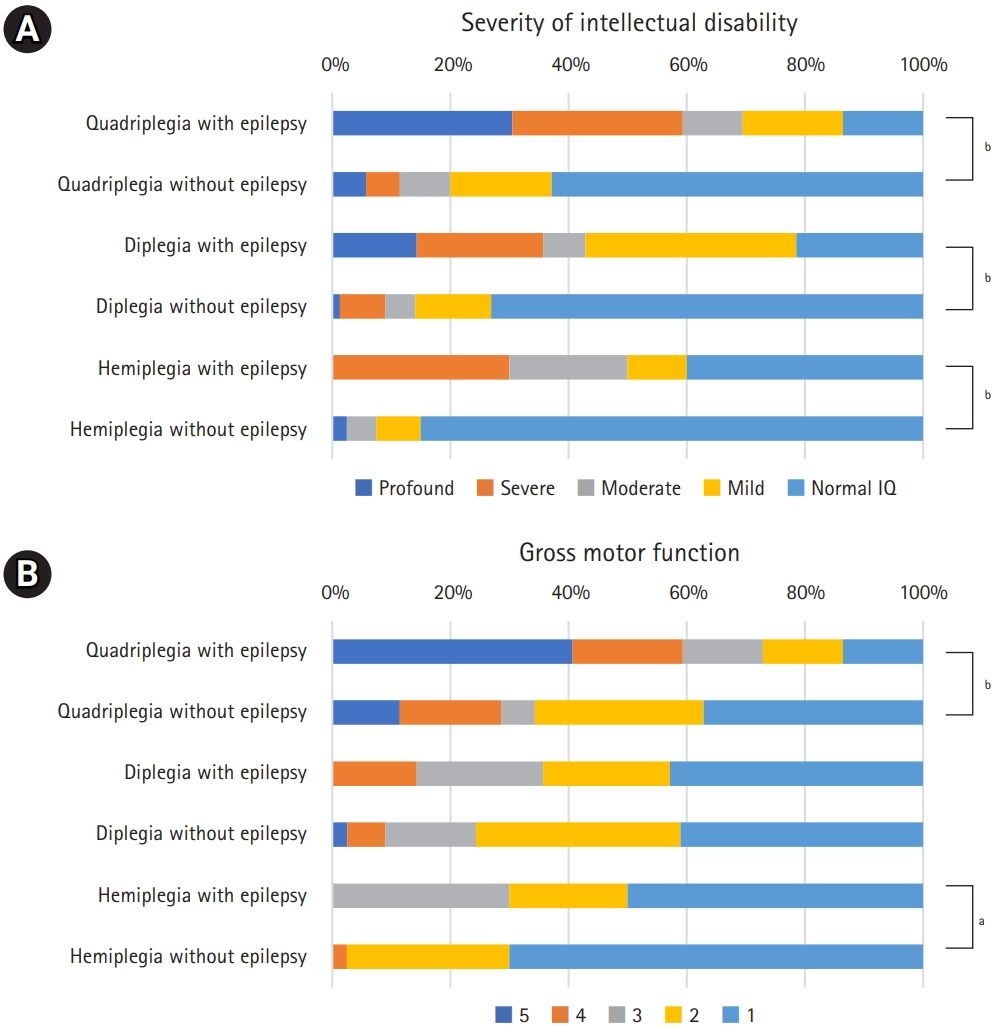
(A) Severity of intellectual disability according to cerebral palsy subtypes. (B) Gross motor function according to cerebral palsy subtypes. IQ, intellectual quotient. aP<0.05; bP<0.01.
The results of comparing the features of DRE and controlled epilepsy are described in Table 3. The most common CP subtype was spastic quadriplegia in both groups (65.5% for DRE, 57.1% for controlled epilepsy), followed by spastic hemiplegia (17.2%) in the DRE group and spastic diplegia (17.1%) in the controlled epilepsy group. The number of patients with ID and severity of ID were comparable in the DRE and controlled epilepsy groups. The DRE group experienced status epilepticus more often than the controlled epilepsy group (13.8% vs. 7.1%, P=0.44), although the difference between the two groups was not statistically significant. The most common brain imaging result in the DRE group was cerebral malformation (31.0%). The occipitofrontal circumferences were significantly different between the two groups, with macrocephaly being present in 27.6% of subjects in the DRE group and 2.9% in the controlled epilepsy group (P<0.001). MRI findings were significantly different between the two subgroups, with DRE patients having higher frequencies of cerebral malformation (31.0% vs. 4.3%) and diffuse gray matter injury (17.2% vs. 11.4%), but lower frequencies of periventricular leukomalacia or periventricular white matter injury (27.6% vs. 41.4%) than controlled epilepsy patients. The most commonly used ASM for monotherapy was levetiracetam (33.3%), followed by valproate (26.7%) and oxcarbazepine (16.7%). For polytherapy, valproate (27.5%) was the most commonly used medication in various combinations with other ASMs, followed by levetiracetam (21.7%). The ASMs used in DRE and controlled epilepsy are shown in Table 4.
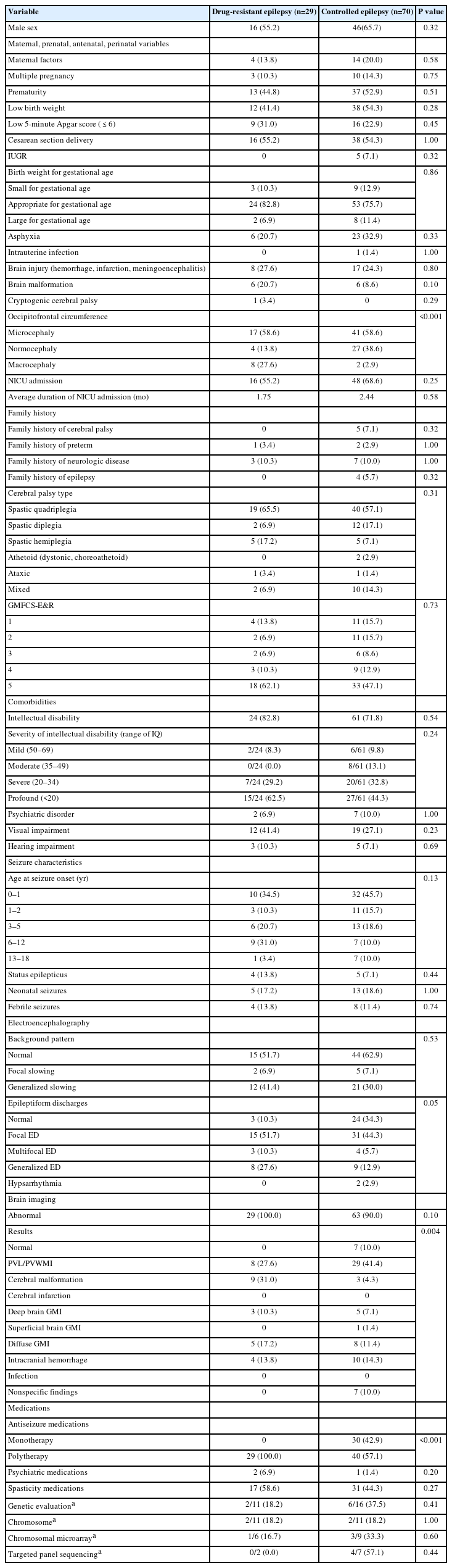
Comparison of demographic and clinical features of cerebral palsy patients with drug-resistant epilepsy and controlled epilepsy
Discussion
The incidence of CP in Korea is 2 to 4 per 100,000 live births. A recent research based on National Health Information Database of Korea showed that the incidence of CP decreased from 4.1 per 100,000 live births in 2007 to 2.5 in 2011 despite an increase in high-risk deliveries [3]. However, some studies in other parts of the world have shown the incidence did not change significantly over time or even increased despite advancement of neonatal care such as hypothermic therapy, magnesium sulfate injections for mothers with preterm labor, and an increased survival rate for extremely preterm babies [13,14]. These conflicting results might have arisen from differences in the time points used for comparison and how the population was defined and compiled for each study.
1. Risk factors for epilepsy in cerebral palsy
CP patients born preterm were more prevalent in the non-epilepsy group. A study in Canada based on CP registry also showed similar results, with more preterm patients with CP in the non-epilepsy group [15]. Studies in Australia, Turkey Poland, and India also reported more preterm patients in the non-epilepsy group, albeit without statistical significance [2,10,16,17]. These results seem to contradict the well-known fact that prematurity is a risk factor for CP [1,4]. A recent study based on the Korean population showed that a shorter the gestational age was associated with a higher risk for both CP and epilepsy [18]. The discordant results between population-based studies and our study or studies using CP registries could be explained by different study designs. Our study and studies based on registries included patients diagnosed with CP, without making a comparison with healthy controls. Term babies who develop CP are more likely to have diffuse brain injury or underlying brain lesions or genetic contributions than preterm babies who can have CP by prematurity itself without diffuse brain injuries, brain lesions, or genetic factors.
Our study showed that asphyxia and brain injury, including brain infection, infarction, and hemorrhage were risk factors for epilepsy in CP patients. This could be explained by the definition of CP, according to which prenatal and perinatal brain insults cause the disease. Microcephaly was the most common finding for occipitofrontal circumference in the epilepsy group, while normocephaly was predominant in the non-epilepsy group. The only study showing microcephaly and epilepsy in CP, by Hanci et al. [2], also showed that microcephaly was a risk factor for epilepsy. Whether acquired brain injury or underlying genetic contributors directly caused microcephaly (primary microcephaly) or epilepsy due to brain injury consequently resulted in microcephaly (secondary microcephaly) was not explained. This needs to be further investigated.
More than half of the patients in the epilepsy group had severely impaired gross motor function, while more than half of non-epileptic CP patients had mild gross motor function impairment. The CP registries of Australia and Canada showed similar results, with GMFCS-E&R levels 4 and 5 being present in 25% to 39% of the epilepsy group and 7% to 20% of the non-epilepsy group [10,15]. A study in Turkey showed opposite results, with GMFCS-E&R levels 4 and 5 being present in 34% of the epilepsy group and 64% of the non-epilepsy group [2]. The finding of severely impaired gross motor function in the non-epilepsy group of the Turkish study could be explained by different compositions of CP subtypes, with a higher proportion of spastic quadriplegic patients (39%) but a lower portion of spastic hemiplegic patients (12%) than in our study and other studies.
More CP patients with epilepsy had a history of neonatal seizures than non-epilepsy patients. Previous studies by Karatoprak et al. [6], Kulak and Sobaniec [16], and El-Tallawy et al. [7] also showed that neonatal seizures were a risk factor for epilepsy. An association between epilepsy and history of febrile seizures was observed in our study. To the best of our knowledge, this is the first study to show an association between febrile seizures and epilepsy in CP. The overall occurrence of febrile seizures in the total population was 9.0% in this study, which was within the range of febrile seizure occurrence in the general population of children in Asia (8% to 10%) [19]. CP itself does not seem to increase the risk of febrile seizures.
One of four patients in the non-epilepsy group had abnormal EEG results, and all of them were generalized slowing of the background pattern. In previous studies, 20% to 40% of non-epileptic CP patients had abnormal EEG findings, such as focal and generalized epileptiform discharges and abnormalities induced by hyperventilation or photic stimulation [2,20]. Jaseja [21] has proposed that interictal epileptiform discharges can affect cognitive function and that slow wave activities are associated with memory impairment in CP patients. Our study included only a limited number of EEG examinations for non-epilepsy CP patients. Whether abnormal findings on EEG of non-epilepsy CP patients have a predictive value for function or the future onset of epilepsy remains unclear. Further studies analyzing EEG signals might answer this question.
Our study results showed that 10.1% of CP patients had normal brain imaging results, similar to the results of previous studies, showing that 7% to 17% of patients with CP had normal brain imaging findings [1,5,6,20,22]. Reid et al. [23] showed that generalized cortical-subcortical involvement and white matter loss were associated with epilepsy in CP patients. For both the epilepsy and non-epilepsy groups, periventricular leukomalacia or periventricular white matter injury was the most prevalent (37.4% and 58.4%, respectively). Although brain imaging results were categorized differently, Karatoprak et al. [6] showed that predominant white matter injury was the most common finding in both the epilepsy and non-epilepsy groups. The number of categories for classifying brain imaging was as small as 6 to as many as 20 among different studies [5,6,20,22,23]. To precisely predict the prognosis using the results of brain imaging, a more consistent classification for imaging in CP patients should be established.
Previously, perinatal hypoxia was thought to be a major cause of CP. However, recent studies have shown that it is only responsible for about 10% of CP cases [1,24]. CP patients without perinatal risk factors (cryptogenic or idiopathic CP) or the presence of any red flag signs are encouraged to receive genetic evaluations. Red flags include consanguinity, congenital anomaly, dysmorphism, normal brain MRI findings, a mismatch between perinatal history and MRI findings, and more than one affected family member [25]. In an unselected CP cohort, 6% to 45% of patients were detected by exome sequencing and 4% to 10% were detected by a chromosomal microarray [25-27]. The diagnostic yield for cryptogenic CP patients increases if genetic tests are done: 14% to 53% for exome sequencing and 9% to 31% for chromosome microarray [24-26,28]. The diagnostic yield also increases in CP patients with ID, both ID and epilepsy, and both ID and ASD [24]. Patients with congenital anomalies or major dysmorphic features more frequently had copy number variations than single-nucleotide polymorphisms [29]. CP patients without obvious perinatal risks or with concomitant ID can be candidates for genetic evaluation. Discovering monogenic contributors and identifying susceptibility genes should parallel the advancement of genotype-phenotype correlations.
2. Subgroup analysis by subtypes of cerebral palsy
Since CP is a heterogeneous condition, some studies on CP were done by dividing patients into groups based on CP subtypes [10,30] or choosing one subtype of CP (i.e., hemiplegia) [31]. Our study showed a strong association of CP subtypes with epilepsy, leading to a subgroup analysis based on CP subtypes. Risk factors such as prematurity and asphyxia identified in the total group showed increased prevalence in patients with epilepsy among various subtypes. However, statistical significance was not reached due to the small sample size. Differences in the severity of ID and level of gross motor dysfunction were preserved in the subgroup analysis despite a decreased sample size. A comparison of age at seizure onset between subgroups showed differences between spastic quadriplegia and hemiplegia, spastic diplegia, and hemiplegia. Kulak and Sobaniec [16] showed that 68% of spastic quadriplegia patients had an onset of epilepsy during the first year of life. Since the degree of ID and gross motor function differ by CP subtypes, stratifying CP patients according to the CP type might be needed in research on prognostic factors and comorbidities.
3. Subgroup analysis by drug resistance
It was found that 36.9% and 10.8% of CP patients had controlled epilepsy and DRE, respectively. Previous studies showed that 10% to 27% of CP patients had drug resistance [2,5,7-10,17]. The risk factors for drug-resistant and controlled epilepsy were similar for most variables, except for occipitofrontal circumference (P<0.001) and brain imaging findings. A univariate comparison by Hanci et al. [2] showed that asphyxia, CP type, gross motor function, and severity of ID were comparable between drug-resistant and controlled epilepsy groups. Focal and generalized epileptiform discharges were associated with DRE [2]. A 5-minute Apgar score less than 5, neonatal seizures, focal onset seizures, and focal slowing on EEG were risk factors for DRE in the study of Tokatly Latzer et al. [32].
A study in Australia on epilepsy among hemiplegic CP patients showed that 35% of patients were controlled with monotherapy and two-thirds were taking at least two ASMs [10]. Valproate, carbamazepine, and levetiracetam were frequently prescribed ASMs. Changing carbamazepine to oxcarbazepine, our patients also had these three drugs in the top prescribed ASMs. In Sweden, the most commonly used ASM was valproate followed by oxcarbazepine in monotherapy, and valproate followed by levetiracetam in polytherapy [30].
Our study identified risk factors for epilepsy and DRE in CP patients. Different subtypes of CP showed differences in clinical features, risk factors, and functional outcomes. However, our study has some limitations. Most importantly, this was a retrospective study based on medical records. Second, recall bias of information related to birth existed for patients born from a different hospital. A prospective study enrolling newborns, collecting data related to risk factors for CP, and developing a prediction model for future occurrence of seizure or epilepsy will help parents and physicians to stratify and monitor CP patients for seizures.
Conclusion
CP patients with asphyxia, hemorrhage, infarction, and brain infections were more likely to have epilepsy. Spastic quadriplegia was the most common subtype of CP among patients with epilepsy. Epileptic CP patients were more likely to have neonatal seizures with more severely impaired gross motor function, and ID. Stratifying by subtypes is encouraged for further research on the etiology of CP, comorbidities in CP patients, and their prognosis.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.26815/acn.2023.00220.
Subgroup analysis for epilepsy risk factors. IUGR, intrauterine growth retardation. aP<0.05; bP<0.01.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JYH and JMK. Data curation: HY and JMK. Formal analysis: JYH and JMK. Funding acquisition: JMK. Methodology: JYH. Project administration: JMK. Visualization: JYH. Writing-original draft: HY and JMK. Writing-review & editing: HY, JYH, and JMK.
Acknowledgements
This work was supported by The Catholic University of Korea, Daejeon St. Mary’s Hospital, Clinical research institute Grant funded by The Catholic University of Korea, Daejeon St. Mary’s Hospital (CMCDJ-P-2023-009).