Assessment of Sleep Disorders in Children with Transfusion-Dependent Hemoglobinopathies
Article information
Abstract
Purpose
The aim of this study was to compare sleep problems between children with transfusion-dependent hemoglobinopathies and healthy controls.
Methods
This study was a case-control survey of children with transfusion-dependent hemoglobinopathies. The sample consisted of 175 children in the patient group and 175 healthy children in the control group, with an age range of 8 to 18 years. Subjects were recruited from the Children's Hospital of Mansoura University between February and July 2022. Children with transfusion-dependent hemoglobinopathies received consultations at the Department of Pediatric Hematology. The Children’s Sleep Habits Questionnaire (CSHQ) was used to evaluate sleep problems in both groups.
Results
The mean age of the patient group was 11.22±2.39 years, and 52.57% (n=92) were girls. The control group had a mean age of 11.30±2.16 years, and 50.86% (n=89) were boys. The overall score (P=0.007) and the night waking (P=0.013), sleep duration (P=0.009), and sleep-disordered breathing (P=0.029) subscores were all substantially and statistically significantly higher in children with transfusion-dependent hemoglobinopathies than in healthy children.
Conclusion
As children with transfusion-dependent hemoglobinopathies have more sleep problems than healthy children, more detailed studies are needed.
Introduction
Hereditary hemoglobin diseases, often known as hemoglobinopathies, are a set of hereditary illnesses involving the structure of hemoglobin. Sickle cell disease (SCD) and thalassemias constitute the majority of these conditions [1,2]. These illnesses are among the most prevalent inherited conditions in the world; the birth rate of individuals who are homozygous or compound heterozygous for symptomatic hemoglobin disorders is around 2.4 per 1,000 births, of whom 1.96 have SCD and 0.44 have thalassemia [3,4].
A β-globin gene mutation produces sickle hemoglobin, which fills red blood cells and changes their shape and flexibility (the "sickling" process). Exercise and oxidative stress enhance cellular dehydration. Hemoglobin deoxygenation and the intracellular sickle cell hemoglobin concentration affect sickling, polymerization, and severity [5,6]. A vaso-occlusive or hemolytic pathophysiology generates acute and persistent SCD symptoms [7,8]. Vaso-occlusive disease causes chest discomfort and osteonecrosis, while hemolysis causes pulmonary hypertension, priapism, and leg ulcers [9,10]. Thalassemias are hereditary. Hemoglobin develops with two β-chains and two α-chains [11,12]. In α-thalassemia, the α-globin gene cluster is altered, whereas beta-thalassemia involves mutations in the gene coding for β-globin. Depending on normal hemoglobin, thalassemia may range from almost asymptomatic to severe anemia needing lifelong blood transfusions and organ damage [13].
Transfusion-dependent hemoglobinopathies cause nocturnal oxyhemoglobin desaturation in 40% of affected children [14]. Oxyhemoglobin levels may drop significantly in children with transfusion-dependent hemoglobinopathies compared to healthy controls, and the severity of SCD is proportional to the extent of desaturation. Daytime hemoglobin saturation is lower in sickle cell anemia patients, lowering their blood oxygen levels. Adenotonsillar hypertrophy-related obstructive sleep apnea (OSA) or SCD-related chronic lung illness may cause hypoxemia [15].
Sleep-disordered breathing occurs when nightly ventilation patterns or amounts are incorrect. OSA, the most common type of sleep-disordered breathing, occurs when the upper airway collapses, reducing airflow and causing hypoxia, sleep disruptions, hypercapnia, and neurocognitive impairment [16]. Snoring, the most common symptom of upper airway resistance, causes sleep-disordered breathing in 7% to 13% of healthy children ages 2 to 8 and 3% to 5% of older children. Sleep-disordered breathing may cause behavioral challenges, learning difficulties, pulmonary hypertension, arterial hypertension, nocturnal enuresis, and developmental concerns in children [17].
Oxygen desaturation below 85% was found to be four times more likely in people with transfusion-dependent hemoglobinopathies and sleep apnea than in people with SCD and no sleep apnea [18]. A spleen injury during childhood may cause enlarged tonsils and adenoids, which may restrict airflow and cause sleep-disordered breathing. These alterations in nocturnal respiration may cause nocturnal hypoxemia due to SCD-enhanced sleep-disordered breathing [19].
Sleep is one of the essential daily requirements for humans. Sleep promotes cellular regeneration, relaxation, comfort, and physical-mental rest. Numerous studies have shown that sleep disturbances are particularly common in sick people [20]. Children with chronic illnesses are affected by a variety of physical and behavioral issues, including sleep disturbances. Chronic illnesses are a leading cause of disability and death. Studies have shown that 30% to 75% of newly diagnosed cancer patients experience a range of sleep difficulties [21]. These patients' sleep disturbances result in chronic weariness, decreased adherence to therapy, and a diminished quality of life [22].
Only 17% of people with a sleep disturbance are checked and treated by doctors, despite the significance of sleep quality [23]. Transfusion-dependent hemoglobinopathies cause various psychological and physical difficulties in sufferers. In particular, these patients often experience a high degree of worry, which disrupts their sleep [24]. However, little research has been undertaken on this topic [25].
Sleep-related disorders are sleep-pattern-altering abnormalities that result in inadequate or poor-quality sleep. Sleep-disordered breathing (e.g., OSA and upper airway obstruction), restless legs syndrome, and insomnia are sleep disorders that make it difficult to begin or sustain high-quality sleep [26]. In the general pediatric population, sleep difficulties are linked with poor effects on learning and attention, diminished cognition, behavioral issues, and reduced health-related quality of life [21].
Considering the high prevalence of snoring and other sleep problems in children with transfusion-dependent hemoglobinopathies and given the time constraints of busy pediatricians to screen for sleep disorders, screening tools have been developed to assist pediatricians in recognizing these symptoms [27]. These questionnaires are comprehensive and are capable of predicting the probability of sleep disorders with a relatively high degree of accuracy [28].
A polysomnographic study (PSG) is the diagnostic technique of choice for quantifying sleep-disordered breathing and sleep architecture. PSG can be performed in children of all ages [29]. PSG is not only useful for diagnosis, but also allows the severity of the disease to be estimated, despite certain limitations: PSG is intrusive, cumbersome, and cost-prohibitive, and facilities that can provide PSG-based diagnostic services in children are not widely available. Furthermore, normative values are based on the statistical distribution of data, and to date, abnormal PSG variables seen in children with OSA have failed to predict adverse clinical outcomes [30].
Given the limitations of PSG, several screening studies have been suggested as a means to distinguish primary snoring and OSA. These studies are based on the frequency and severity of the three cardinal symptoms of sleep apnea: snoring, labored breathing, and oxygen desaturation [31]. Nap studies and overnight oximetry studies have high specificity but low sensitivity; thus, overnight PSG is needed to make the diagnosis in children with negative studies. The other limitations of screening studies include difficulty in estimating the severity of the disease, inability to exclude causes of hypoxemia other than OSA, and an inability to diagnose upper airway resistance syndrome and obstructive hypoventilation, which are other variants of sleep-disordered breathing in children [32].
To the best of our knowledge, existing research on the sleeping patterns and behaviors of children and adolescents with transfusion-dependent hemoglobinopathies, which cause chronic hemolytic anemia, is inadequate [33]. This study intended to compare sleep difficulties between children with transfusion-dependent hemoglobinopathies and healthy controls [34].
Materials and Methods
1. Aim of the study
The aim of this study was to compare sleep problems between children with transfusion-dependent hemoglobinopathies and healthy controls.
2. Research design
A case-control design was used in this study.
3. Study subjects
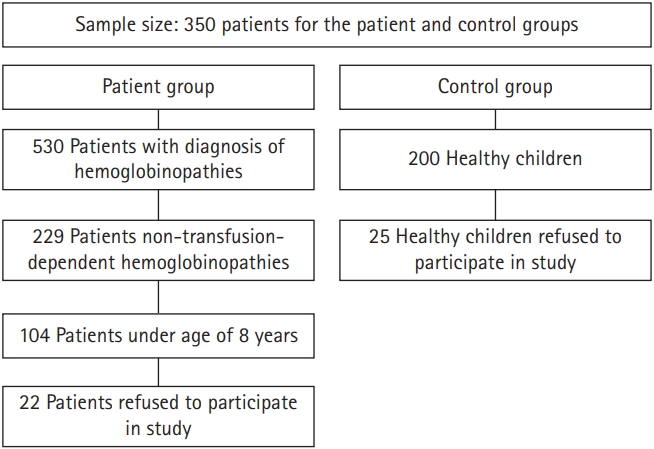
A convenience sample of 175 children with transfusion-dependent hemoglobinopathies who attended the inpatient hematology department and outpatient hematology clinic of the Children's Hospital of Mansoura University was selected for this study, and 175 healthy controls completed the study requirements and were included in the study. In total, 405 children, ranging in age from 8 to 18 years, attended the above-mentioned departments during the 6-month period from February 1, 2021 to July 31, 2022. An assessment for hemoglobinopathy often comprises tests that identify the different forms of hemoglobin and quantify their levels. The information obtained from these tests, in conjunction with the outcomes of standard tests such as a complete blood count, a blood smear, and electrophoresis of globin proteins, helps to reach a diagnosis. There are two categories of children who are diagnosed with hemoglobinopathies: those who need regular blood transfusions (transfusion-dependent) and those who do not (non-transfusion-dependent).
The subjects of this study were required to meet the following criteria (Fig. 1):
(1) Age: between 8 and 18 years
(2) Sex: both sexes
(3) Children who visited the outpatient clinic and inpatient department of the Children's Hospital of Mansoura University.
(4) Children who were blood transfusion-dependent
The exclusion criteria comprised patients with co-morbid illnesses, such as intellectual disability, endocrinological diseases (e.g., diabetes mellitus), cardiovascular diseases, respiratory problems (e.g., asthma), kidney disease (e.g., renal failure), and neurological disorders (e.g., epilepsy and seizures). For context regarding the subjects’ educational level, the public education system in Egypt consists of three levels: the primary educational level for 6 years, starting from 6 to 11 years of age; preparatory school for 3 years, starting from 12 to 14 years of age; and, the secondary school stage for 3 years, from ages 15 to 17 years of age.
4. Data collection tools
Information was collected on children’s sociodemographic characteristics and clinical data using a sheet designed by the researcher based on a review of the literature for the collection of sociodemographic data from children and their caregivers. The variables included (1) sociodemographic data of children (age, sex, birth order, and level of education) and (2) clinical data from children with hematological disorders, such as the onset of the disease, duration of illness, frequency of blood transfusion, the presence of any other disease (e.g., diabetes mellitus, heart disease [cardiomegaly], and bone deformities [fractures or osteoporosis]), and the presence of thalassemia in the family (including the number of brothers or sisters affected by thalassemia and the familial relationship between parents).
The Children's Sleep Habits Questionnaire (CSHQ) is a 45-item parent survey that has been used in past studies to evaluate how children sleep and determine whether they have any problems [35]. The CSHQ includes items on bedtime resistance (items 1, 3, 4, 5, 6, and 8), sleep onset delay (item 2), sleep duration (items 9, 10, and 11), sleep anxiety (items 5, 7, 8, and 21), night waking (items 16, 24, and 25), sleep-disordered breathing (items 18, 19, and 20), parasomnias (items 12, 13, 14, 15, 17, 22, and 23), and morning waking/daytime sleepiness (items 26, 27, 28, 29, 30, 31, 32, and 33). Items are rated on a 3-point scale: "usually" if the sleep behavior occurs five to seven times per week; "sometimes" for two to four times per week; and "rarely" for zero to one time per week. Items 1, 2, 3 10, 11, and 26 are reverse-coded.
Parents retrospectively complete the CSHQ and are asked to assess the child's sleep habits over the previous week. It generally takes 5 to 15 minutes to fill out the questionnaire. A total of 41 points has been suggested as a cut-off point, with higher scores considered to be clinically significant. The Arabic version used in the present work was developed through a translation and retranslation process by Asaad and Kahla [36].
5. Statistical analysis
The collected data were analyzed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Demographic variables were presented using descriptive statistics. Categorical variables were compared using the chi-square test. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to check the normality of data distribution for all continuous variables. Normally distributed parametric variables were compared between groups using the independent-samples t-test. A P value <0.05 was considered to indicate statistical significance.
6. Ethical considerations
Verbal informed consent was obtained from all children and their caregivers. All children and their caregivers were informed of their right to refuse or withdraw at any time. The children's privacy was maintained. Ethical committee approval was obtained from the Research Ethics Committee of the Faculty of Nursing, Mansoura University (MFN-IRB No. 2022-02-0113).
Results
Table 1 shows the frequency distribution of the children according to their sociodemographic and clinical characteristics. Approximately two-thirds (62.86%) of the children in the patient group were in the age group of 8 years to less than 12 years, with a mean age of 11.22±2.39 years. Likewise, almost two-thirds (60.57%) of the children in the control group were in the age group of 8 years to less than 12 years, with a mean age of 11.30±2.16 years. In addition, girls represented a higher percentage (52.57%) in the patient group than in the control group (50.86%). Almost half of the children were first children in both the patient group (41.14%) and the control group (44.57%). Approximately two-thirds of the children were at the primary level in both the patient group (62.86%) and control group (66.29%). Statistically significant differences were not found between the two groups in terms of age, sex, educational level, or birth order.
Table 2 shows the distribution of patients according to their clinical data. More than half of the patients (51.43%) had beta-thalassemia major and a similar percentage (53.71%) had a blood transfusion rate of once monthly. The mean age of children when the disease was diagnosed was 7.49±3.06 months, which was also children’s mean age of children at the time of the first blood transfusion. More than three-quarters of the studied children (77.71%) had a related disease that was detected urgently. Most of the children (80.57%) had splenomegaly, the majority (79.43%) had cardiomegaly, approximately three-quarters of the children (71.43%) had hepatomegaly, over four-fifths of the study children (85.14%) had osteoporosis, and approximately three-quarters (76.0%) had a bone deformity.
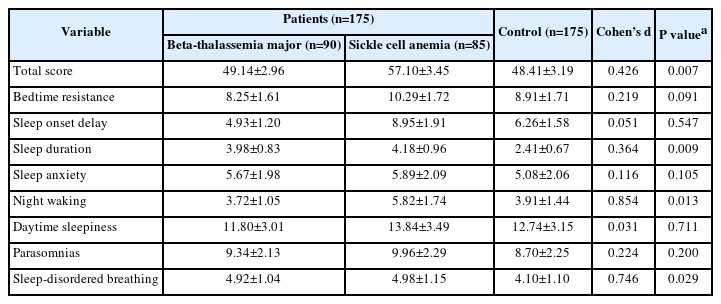
Table 3 presents a comparison of CSHQ ratings between the patient group and the control group. When compared to the control group, the overall score (P=0.007) and the night waking (P=0.013), sleep duration (P=0.009), and sleep-disordered breathing (P=0.029) subscores were all substantially higher in the patient group. Although the patient group had greater levels of bedtime resistance (P=0.091), sleep start delay (P=0.547), sleep anxiety (P=0.105), daytime sleepiness (P=0.711), and parasomnias (P=0.200), these results did not reach statistical significance. Furthermore, higher scores were found for sleep disorders in SCD patients than in patients with thalassemia.
Discussion
In order to address the research gap related to sleep disorders in children with transfusion-dependent hemoglobinopathies, the purpose of the present study was to compare sleep problems between children with transfusion-dependent hemoglobinopathies and healthy controls. According to the comments of the children's parents on the CSHQ scale, the researchers in this study compared the sleeping patterns and behaviors between these two groups of children.
Several reports have described difficulties sleeping among children and adolescents who have hemoglobinopathies. The majority of these findings are linked to SCD, a hereditary hemoglobinopathy that is characterized by persistent hemolysis and increased extramedullary hematopoiesis similar to that seen in thalassemia. However, relatively little research has investigated the connection between thalassemia and sleep disorders [37].
We identified evidence of disturbed sleep in a clinical sample of children with transfusion-dependent hemoglobinopathies. By all measures, the sleep disturbances in our sample, compared to published samples of healthy children, were severe, in agreement with the findings of other studies. In a study conducted by Tarasiuk et al. [38], the authors concluded that children and adolescents with beta-thalassemia or congenital dyserythropoietic anemia type 1 showed impaired sleep function, which was partially related to periodic limb movements and arousals that result in daytime sleepiness. Another study designed by Sritippayawan et al. [39] showed higher prevalence rates of OSA in children with severe beta-thalassemia. Our findings in this study were consistent with the literature. Parents of children with beta-thalassemia major reported more sleep disturbances than those of healthy children. Furthermore, night waking and sleep-disordered breathing were reported to be more common in children with beta-thalassemia major. Reporting these two types of sleep problems together may be an important point, since it may be speculated that sleep-disordered breathing could cause children to wake up more often during the sleep period.
There is a lack of understanding of the mechanism behind sleep disorders in children with beta-thalassemia. Kapelushnik et al. [40] described a child with both OSA and thalassemia intermedia. They hypothesized that the child's OSA was caused by extramedullary hematopoiesis in the nasopharyngeal region. All of the patients in the study by Sritippayawan et al. [39] who had OSA also had adenoid hypertrophy, and 80% of them also had related tonsil enlargement. All of the lymphoid tissues found in the adenotonsillar region had reactive lymphoid hyperplasia in their investigation, but there was no indication of extramedullary erythropoiesis. The authors hypothesized that lymphoid hyperplasia could be connected to recurrent infections of the adenotonsillar tissue, similar to SCD [41].
The limitations of the current study should be taken into consideration when judging the significance of its findings. The fact that we relied on information from the parents is a significant constraint [41,42]. Their interpretation of the child's condition can be colored by the difficulties they are experiencing as parents. For instance, depressed parents may have a more pessimistic outlook on their children’s quality of sleep [43]. Both polysomnography and actigraphy are objective measurements that may be used to precisely assess whether or not a child or teenager is experiencing sleep issues [43,44]. Another disadvantage is that no family or environmental factors were considered, such as marital issues, socioeconomic position, or the setting in which the subject slept. There is a possibility that these factors can also affect young children’s sleep quality [41].
In conclusion, transfusion-dependent hemoglobinopathies may increase the risk of sleep disturbances in children and adolescents, particularly those with SCD rather than thalassemia. Hence, assessing children with transfusion-dependent hemoglobinopathies during their routine clinical check-ups is crucial. Disrupted sleep has been linked to poor cognitive and scholastic performance, behavioral difficulties, and symptoms of depression and anxiety [19-21]. Sleep issues may make it harder for children to function, develop, and manage the main symptoms of beta-thalassemia. For patients with this disease, regular clinical evaluations should examine sleep disorders, including insomnia, nocturnal awakening, sleepwalking, sleep apnea, and daytime sleepiness. Managing children's sleep concerns may also improve therapeutic compliance. Working with parents to improve the sleeping environment may improve sleep quality and quantity (for example, by making the surroundings calmer). Educating parents and children on efficient sleep and bedtime routines at regular healthcare visits may improve children's sleep efficacy.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: MAE. Data curation: MAE. Formal analysis: AMA. Methodology: AMA and ADM. Project administration: AMA. Visualization: ADM. Writing-original draft: AMA. Writing-review & editing: ADM and MAE.