Changes in Sleep Patterns in Korean Early Adolescents during Sexual Maturation
Article information
Abstract
Purpose
Teenagers’ sleep patterns show physiological delays influenced by sexual maturation and other external time-related factors. However, Korean adolescents show differences in the onset of pubertal development and have shorter sleep durations than other adolescents worldwide. Therefore, we assessed sleep patterns and sexual maturation in Korean early adolescents to evaluate changes in sleep patterns in relation to sexual maturation in early adolescents with sleep deprivation.
Methods
From March to August 2017, we surveyed children aged 10 to 12 years in Seongnam (Seongnam Atopy Project). We evaluated items related to sleep and sexual maturation, assessed sleep duration and sleepiness scale scores, and analyzed the relationships of sleep parameters with sex, height, weight, and sexual maturation rating (SMR).
Results
In total, 620 children were included. Sleep duration was 8.63±0.81 hours in boys and 8.40±0.98 hours in girls. Sleep started from PM 11:00±AM 0:47 in boys and PM 11:13±AM 1:06 in girls, and ended at AM 7:38±AM 0:27 in boys and AM 7:34±AM 0:27 in girls. After adjusting for sex and standardized body mass index, bedtime was delayed as the SMR increased (mean delay for each rating increase, 0.251 hours; P=0.001; 95% confidence interval [CI], 0.105 to 0.397). SMR did not influence the wake-up time, although sleep duration decreased as the SMR increased (mean decrease for each rating increase, 0.258 hours; P=0.001; 95% CI, –0.403 to –0.114). The sleepiness scale scores showed no relationship with SMR.
Conclusion
Sleep patterns, especially sleep duration and bedtimes, show changes with sexual maturation in adolescents, who are vulnerable to sleep deprivation.
Introduction
Sleep patterns in adolescents are physiologically delayed [1], and the mechanism underlying these delays is currently unclear. This physiological delay is not caused by environmental factors, such as evening light exposure [2]. Moreover, internal sleep phases in teenagers are delayed compared to those in children, and these delays present as delayed dim light melatonin onset (DLMO) [3]. The interval between DLMO and sleep onset is also prolonged in adolescents [4]. Individuals in the mature Tanner stage show increased sleep latency, delayed bedtime [1,5], and increased sensitivity of the circadian system to light in early puberty [6]. Similar to humans, numerous mammals also show physiological sleep delays in adolescence [7]. In combination with earlier school start times, this physiological delay in sleep can induce sleep deprivation in older adolescents.
Sleep patterns can also be affected by numerous external factors, especially in teenagers. For example, teenagers in East Asia experience more extreme sleep deprivation than those in other parts of the world. Numerous studies have shown that teenagers in Asia have a sleep duration of 6 to 7 hours on weekdays [8], and sleep deprivation becomes more severe as they get older. This pattern of severe sleep deprivation has been attributed to social and academic needs, which are potent regulators of sleep patterns in school children.
The age of onset of sexual development in adolescents in Korea has been reported to be different. Moreover, compared to a study published in 2006 [9], a more recent study from 2020 reported earlier pubertal development onset in Korean adolescents [10]. However, studies on the relationship between the Tanner stage and sleep have not been conducted in Korean adolescents. Therefore, considering the differences in life patterns of East Asian adolescents from those of adolescents in other countries, we believe that the findings of this study will provide useful insights into the relationship between sexual development represented by Tanner stage and sleep patterns in early adolescents, who often experience sleep deprivation.
Materials and Methods
1. Participants
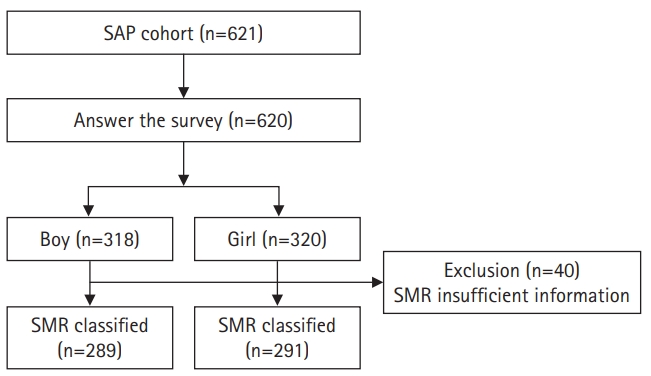
This study surveyed children aged between 10 and 12 years who attended 11 elementary schools in Seongnam from March to August 2017 as part of the Seongnam Atopy Project (SAP). In the SAP, we surveyed the students’ characteristics, body weight and height, date of birth, questionnaires about allergic disease and gastrointestinal disease, routine diet, environmental factors, feeding history, sleep and sexual maturation with figures (Fig. 1). We selected questionnaire items to survey the participants’ sleep patterns, sleepiness, anthropometric data, and baseline characteristics (Appendix 1). We asked the children’s parents to complete the questionnaires prior to physical examinations. The parents of 621 children agreed to participate in this study, and 620 children returned completed demographic questionnaires, including information regarding school year and sex. The physical examination, which included measurement of weight and height, was performed by a pediatrician and a well-trained technician. Body mass index (BMI) was calculated from the measured height and weight and standardized with reference to the age and sex (BMI-z) [11,12]. Sex and BMI are known to affect sleep based on previous studies, so they were used as correction variables.
2. Sleep duration and daytime sleepiness
Participants’ usual sleep onset and wake time for the last 7 days were determined using direct questions. Sleep duration was computed from the sleep onset and wake times. Responses describing unusual times of sleep onset and waking, such as sleep onset in the afternoon, getting up in the evening, or sleep duration over 15 hours, were excluded. The degree of sleepiness was estimated using the Korean version of the Pediatric Daytime Sleepiness Scale (PDSS) [13]. The PDSS has eight questions, each of which is scored from 0 to 4 (total range, 0 to 32), and it assesses subjective sleepiness during classes, homework, daytime, and morning. A higher score indicates greater daytime sleepiness [13], and a score of 15 or more in the age group of 14 to 19 years old indicates excessive sleepiness [14].
3. Sexual maturity
The Tanner stage is a common parameter for evaluating children’s pubertal development, which is classified using the sexual maturation rating (SMR) scale [15]. Parents answered questionnaires about their children’s sexual maturity, which included pictures with serial stages of sexual maturity. The series of pictures outlined the degrees of development of pubic hair and breasts in girls and pubic hair, penis, and testes in boys. On the basis of the responses, for boys, an SMR of 2 implied visible signs of testicular enlargement or development of pubic hair; an SMR of 3 indicated penile growth or further development of pubic hair; and an SMR of 4 indicated increased testes volume or a distributed pattern of pubic hair. For girls, an SMR of 2 indicated the presence of breast buds or development of pubic hair, and SMRs of 3 to 5 indicated patterns of pubic hair and breast development. The selected pattern of SMR was different; the average grade was used, whereas in cases with only one response for the queries regarding sexual development, the SMR was classified on the basis of this response.
4. Ethics statement
The survey was approved by the Institutional Review Board of CHA Bundang Medical Center (IRB No. 2017-04-049). Written informed consent was obtained from the parents or caregivers of all participants.
5. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) and MS Excel version 2203 (Microsoft, Redmond, WA, USA). Frequencies and continuous variables were compared using the chi-square test and analysis of variance, respectively. Multiple linear regression models were used to estimate adjusted differences and 95% confidence intervals (CIs), with adjustments for sex and BMI-z. Sex and BMI have previously been reported as factors that influence sleep patterns and obstructive sleep apnea syndrome [15,16]. Each sleep-related variable was analyzed in a model with SMR, sex, and BMI-z. Statistical significance was defined as a P<0.05.
Results
1. Sample characteristics
We evaluated the findings for 620 children (318 boys and 302 girls) who provided basic data such as sex and grade/school year. The mean ages were 11.51±0.61 years for boys and 11.48±0.90 years for girls. The 318 boys included four (1.3%) fourth-grade boys, 154 (48.6%) fifth-graders, and 159 (50.2%) sixth-graders, while the 302 girls included two (0.7%) fourth-graders, 152 (50.3%) fifth-graders, and 148 (49.0%) sixth-graders.
The mean heights were 149.15±8.05 cm for boys and 149.43±7.29 cm for girls, and the mean weights were 42.79±9.61 kg for boys and 42.15±8.57 kg for girls. The mean BMI was 19.1±3.27 kg/m2 for boys and 18.77±3.01 kg/m2 for girls, and the mean BMI-z was –0.08±1.01 for boys and 0.03±1.04 for girls.
2. Sleep patterns and sleepiness
We investigated sleep patterns and sleepiness using the Korean version of the PDSS. The mean sleep duration was 8.63±0.81 hours for boys and 8.4±0.98 hours for girls, as calculated based on the bedtime (PM 11:00±AM 0:47 for boys and PM 11:13±AM 1:06 for girls) and wake time (AM 7:38±AM 0:27 for boys and AM 7:34±AM 0:27 for girls). The average PDSS score was 11.4±5.02 for boys and 11.65±5.24 for girls.
3. Sexual development
Among the 318 boys, 289 provided responses for pubic hair and 282 provided responses for genitalia, and the SMR was evaluated in 289 boys. Among the 302 girls, 287 provided responses for pubic hair and 291 provided responses for breasts, and the SMR was evaluated in 291 girls. Among the boys, 237 (82%), 42 (14.5%), and 10 (3.5%) were classified as SMR1, SMR2, and SMR3, respectively. None of the boys were categorized as SMR4. Among the girls, 102 (35.1%), 152 (52.2%), 31 (10.7%), and six (2.1%) were classified as SMR1, SMR2, SMR3, and SMR4, respectively (Table 1).
4. Sleep duration and sleepiness in relation to SMR
Table 2 presents the sleep duration data for the 381 children who answered the survey for sleep patterns. Sleep duration in adolescents was inversely proportional to the SMR: 8.65±0.81 hour/night in SMR1, 8.51±0.80 hour/night in SMR2, 7.89±1.02 hour/night in SMR3, and 7.67±1.26 hour/night in SMR4 (Table 2). Similarly, the bedtime was PM 10:58±AM 0:49 in SMR1, PM 11:07±AM 0:48 in SMR2, PM 11:42±AM 1:02 in SMR3, and PM 11:55±AM 0:52 in SMR4. The wake times were similar and did not differ significantly among participants with different SMRs (P=0.616). The scores on the Korean version of the PDSS tended to increase with the SMR, but the differences were not significant: 11.44±5.04 in SMR1, 11.34±5.10 in SMR2, 12.05±5.50 in SMR3, and 14.50±3.99 in SMR4 (P=0.423). Even when the differences were analyzed based on values of 15 (the cutoff value of 14 to 19 years) [14] and 11 (the average value of the PDSS), no statistically significant results were obtained.
As shown in Table 3, after adjusting for sex and BMI-z, each rating increase in the SMR resulted in a delay of 0.251 hours in bedtime (95% CI, 0.105 to 0.397 hours; P=0.001), no significant changes in the wake time (P=0.817), a reduction of 0.258 hours in sleep duration (95% CI, –0.403 to -0.114 hours; P=0.001), and no significant change in the PDSS score (P=0.310).
Discussion
This study evaluated sleep patterns according to sexual maturation during early puberty in adolescents, who often experience sleep deprivation compared to the recommended sleep duration [16]. The sex- and BMI-adjusted findings suggested that sleep onset was delayed and sleep duration decreased with development of sexual maturity. However, daytime sleepiness showed no significant changes related to SMR. This study is the first to investigate the effect of sexual maturation in Asian adolescents, who often experience sleep deprivation.
Several previous studies have reported changes in sleep duration in adolescence. Rutter et al. [17] reported that sleep duration decreased according to the Tanner stage, after adjusting the findings for other confounding factors. Our findings showed that the total sleep duration per day decreased by approximately 0.258 hours for each stage increase in the SMR. Although this study had fewer adolescents with a mature SMR, it showed a similar pattern of reduction in sleep duration as other studies (0.27 to 0.33 hours per Tanner stage) [17,18]. However, a few studies did not observe significant differences in sleep duration in relation to the Tanner stage [19]. The reasons underlying these discrepancies across different studies are unclear.
Our findings also showed delayed sleep onset as the SMR increased. Delayed sleep onset manifests as a phase delay in the circadian timing system. Although such phase delays are known to be associated with pubertal and adolescent development, the underlying mechanism is unknown, and several attempts have been made to identify this mechanism. The intrinsic period of the circadian timing system may slow through puberty in adolescence, thereby delaying the circadian phase and sleep onset. This may be due to the increased sensitivity of light during DLMO [6]. Another study reported that human adolescents show a phase delay in DLMO according to age [4] and sexual maturation [5]. Delayed sleep onset in adolescence may also be caused by increased sleep latency [1,6]. Changes in phase-dependent sensitivity to light may cause phase delays, with strengthening of the delayed response to evening light and weakening of the advance response to morning light. Regarding DLMO, since homeostatic sleep pressure accumulates faster in younger adolescents than in older adolescents, sleep onset after DLMO is faster [4]. Therefore, the older one gets, the slower sleep onset becomes. Our study also showed that sleep onset was delayed by approximately 0.25 hours for each rating increase in SMR, supporting the findings of previous studies.
We did not observe significant differences in sleepiness according to the SMR. Delayed sleep onset causes a reduction in total sleep duration due to a fixed awake time, and various studies have shown sleepiness in individuals with a higher SMR [20]. Carskadon et al. [19] reported that teenagers in the higher SMR group were sleepier and presented short sleep latency. Another study also showed that the prevalence of excessive daytime sleepiness increased with advanced pubertal maturation [21]; however, a longitudinal cohort study reported that subjective sleepiness was more closely related to age than to pubertal development [22]. Our study included early adolescents with the same school hours, which are known to be one of the most influential factors for sleep [23]. Therefore, we suggested that our findings regarding the effect of sexual maturation on sleepiness in early adolescents are more relevant than those of previous studies.
Sleep deprivation may blunt the effects of sexual maturation on teenagers’ sleepiness. Multiple previous studies have reported that Korean and East Asian teenagers have severely limited sleep due to academic reasons [23]. These patterns have also been observed in elementary school students [8,13]. Age has been reported to cause sleepiness in various studies [13,18,20]. Moreover, age or school year can influence sleep duration due to the school start time. Under conditions of sleep deprivation, age and school start time could have a greater influence on sleepiness than sexual maturation.
This study had several limitations. Although the inclusion of children with SMR1, SMR2, and SMR3 allowed us to evaluate the effects of sexual maturation in the early stages of puberty, it may have resulted in an underestimation of the effect of later SMR stages. In addition, we did not analyze adolescents’ chronotype (i.e., eveningness or morningness), which is also a factor influencing sleepiness in adolescents [24]. Moreover, we were not able to observe differences in the wake time according to the SMR, and this survey was conducted in a single season with participants who had a similar school start time.
In conclusion, sleep onset is delayed and sleep duration decreases with sexual maturation. However, this study found that puberty-related changes in sleepiness did not appear in early puberty or in adolescents, who often experience sleep deprivation. Understanding the physiological delays in sleep related to puberty will be helpful for improving teenagers' quality of life.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: TH and SR. Data curation: EKH, MYH, and HMJ. Formal analysis: TH and SR. Funding acquisition: EKH, MYH, and HMJ. Methodology: TH and SR. Project administration: MYH, HMJ, and SR. Visualization: TH and SR. Writing-original draft: TH. Writing-review & editing: TH, KYC, EGY, MKJ, and SR.
Acknowledgements
This study was supported by the Seongnam Atopy Project (SAP2017). Grant funding was provided by the metropolis of Seongnam.