Cannabidiol Treatment for Lennox-Gastaut Syndrome at a Single Tertiary Center in South Korea
Article information
Abstract
Purpose
This study evaluated the efficacy and tolerability of cannabidiol (CBD) as an add-on therapy for childhood-onset Lennox-Gastaut syndrome (LGS).
Methods
This retrospective study enrolled patients who visited the Department of Pediatric Neurology at Asan Medical Center from March 2019 to February 2022 and were treated with CBD. Electronic medical records and clinically relevant factors (including the type of epilepsy and seizures, etiology, and the number of concomitantly used anti-epileptic drugs) were reviewed. The outcome was clinical response to CBD (≥50% or <50% seizure reduction at 1, 3, and 6 months after CBD introduction and the last follow-up visit). Relevant adverse events were monitored.
Results
Thirty patients were included. The median age of epilepsy onset was 5.5 years (interquartile range [IQR], 3.3 to 25.3), with a median treatment duration of CBD of 6 months (IQR, 3.3 to 7.0). Sixteen patients (53.3%) showed ≥50% seizure reduction at the last follow-up. In a univariate analysis, patients whose epilepsy commenced after 3 years of age were more likely to respond to CBD (odds ratio, 10.11; 95% confidence interval, 1.05 to 97.00; P=0.04). Adverse events were observed in 11 patients (36.6%); the most common adverse event was somnolence.
Conclusion
CBD could be a treatment option for children and young adults with drug-resistant LGS with a tolerable safety profile. Age at epilepsy onset (>3 years) was associated with a favorable response to CBD treatment. Further prospective studies with larger populations are needed to evaluate the tolerability and efficacy of CBD in patients with drug-resistant epilepsy of various etiologies.
Introduction
In recent years, there has been a growing interest in the treatment of epilepsy with medicinal cannabis. Cannabis plants contain more than 100 terpenophenolic compounds, termed phytocannabinoids. The two main compounds are delta-9-tetrahydrocannabinol (delta-9-THC) and cannabidiol (CBD) [1,2]. Delta-9-THC partially activates the cannabinoid receptor type 1 (CB1)/cannabinoid receptor type 2 (CB2) receptor and has a potent anti-inflammatory effect. However, it has psychoactive, cognitive, and behavioral side effects. In contrast to THC, CBD has a very low affinity for CB1 and CB2 receptors, and this means that it lacks a psychoactive effect. Therefore, CBD has been suggested as a treatment for epilepsy that does not involve the endocannabinoid system [3,4].
Medical cannabinoids have been administered to a large number of patients since 2018, and four randomized controlled studies conducted with plant-derived pharmaceutical CBD (Epidiolex, Greenwich Biosciences Inc., Carlsbad, CA, USA) showed meaningful seizure frequency reduction, acceptable tolerability, and safety in patients with Lennox-Gastaut syndrome (LGS), Dravet syndrome (DS), and tuberous sclerosis complex (TSC) [5-10].
In the clinical setting in Korea, a medical cannabis product named Epidiolex (CBD, 100 mg/mL) was approved for use in March 2019 by the Health Insurance Review and Assessment Service and has been administered to patients with LGS and DS aged ≥2 years. Moreover, since April 2021, the National Health Insurance System has covered the expense of CBD for patients with LGS/DS who do not respond to multiple anti-seizure medication (ASM) treatments (over five ASMs), and CBD is, at present, more easily available for many patients with drug-resistant LGS and DS.
This retrospective study was conducted at a single tertiary center in Seoul, South Korea, and aimed to investigate the real-world efficacy of CBD in pediatric and young adult patients. This study aimed to evaluate the efficacy and tolerability of CBD as an add-on therapy for children, adolescents, and young adult patients with drug-resistant LGS and DS.
Materials and Methods
1. Patient population and data collection
This retrospective study was performed on patients with LGS and DS who were treated with Epidiolex (CBD, 100 mg/mL) from March 2019 to February 2022 at Asan Medical Center, Seoul, Korea. Patients treated with CBD for less than 1 month were excluded because the treatment period was insufficient to evaluate efficacy. Clinical data based on electronic medical records such as past medical history, type of seizures, frequency of monthly seizures, underlying etiology, brain magnetic resonance imaging findings, inter-ictal electroencephalography (EEG) abnormalities, the number of concomitantly used ASMs, clinical response, and any adverse events or positive effects reported by parents were reviewed. Seizure type was classified based on the initial manifestation of the seizure as generalized, focal, or combined (if patients had both generalized and focal seizures).
In the clinical setting, CBD was initiated at 2.5 mg/kg orally twice a day. If the patient tolerated the treatment for a week, the dose was increased to 5 mg/kg twice a day, followed by a maintenance dose of 10 to 25 mg/kg/day.
To evaluate seizure outcomes with CBD, data on baseline seizure type and frequency were collected 28 days prior to CBD initiation, and seizure type and frequency were evaluated during the treatment period (1, 3, and 6 months after CBD initiation, as well as at the last follow-up). These observations were documented by the patient’s caregiver or two pediatric neurologists. Response to CBD was defined by a decrease of ≥50% in seizure frequency.
This study was approved by the Institutional Review Board (20180021) of Asan Medical Center. Written informed consent by the patients was waived due to a retrospective nature of our study.
2. Statistical analysis
The data were analyzed using SPSS statistical software version 21.0 (IBM Corp., Armonk, NY, USA). The chi-square and Fisher exact tests were used to analyze qualitative variables. Mean values were compared using the independent t-test for parametric continuous variables, and median values were analyzed using the Wilcoxon rank-sum test for non-parametric continuous variables. The data were presented as mean±standard deviation or median (interquartile range [IQR]). Differences were considered statistically significant at P values <0.05. Univariate logistic regression analysis was performed to identify the potential beneficial factors associated with CBD treatment.
Results
Thirty patients with LGS were included in this study. The demographic findings are summarized in Table 1. CBD treatment was initiated at a mean age of 15.0±6.8 years, with a mean duration of epilepsy treatment of 12.7±6.2 years. The study group consisted of 19 pediatric patients aged <18 years old and 11 adult patients. The onset of epilepsy occurred at 5.5 years (IQR, 3.3 to 25.3), and the patients concomitantly used multiple ASMs (5; IQR, 4.0 to 5.0) and had been previously treated with non-pharmacotherapy (corpus callosotomy, vagal nerve stimulation, and diet therapy). Unknown etiologies were most common (18/30, 60%), followed by structural brain lesions (9/30, 30%), genetic etiologies (2/30, 6.7%), and infectious etiologies (1/30, 3.3%). Twenty patients had a history of infantile spasms, and 22 showed neurocognitive deficits.
The final dosage of CBD was 9.7 mg/kg/day (IQR, 5.2 to 12.8) for 6 months (IQR, 3.3 to 7.0). Adverse events were observed in 11 of 30 (36.6%) patients; the most common adverse event was somnolence, followed by diarrhea, speech disturbance, and gait abnormality.
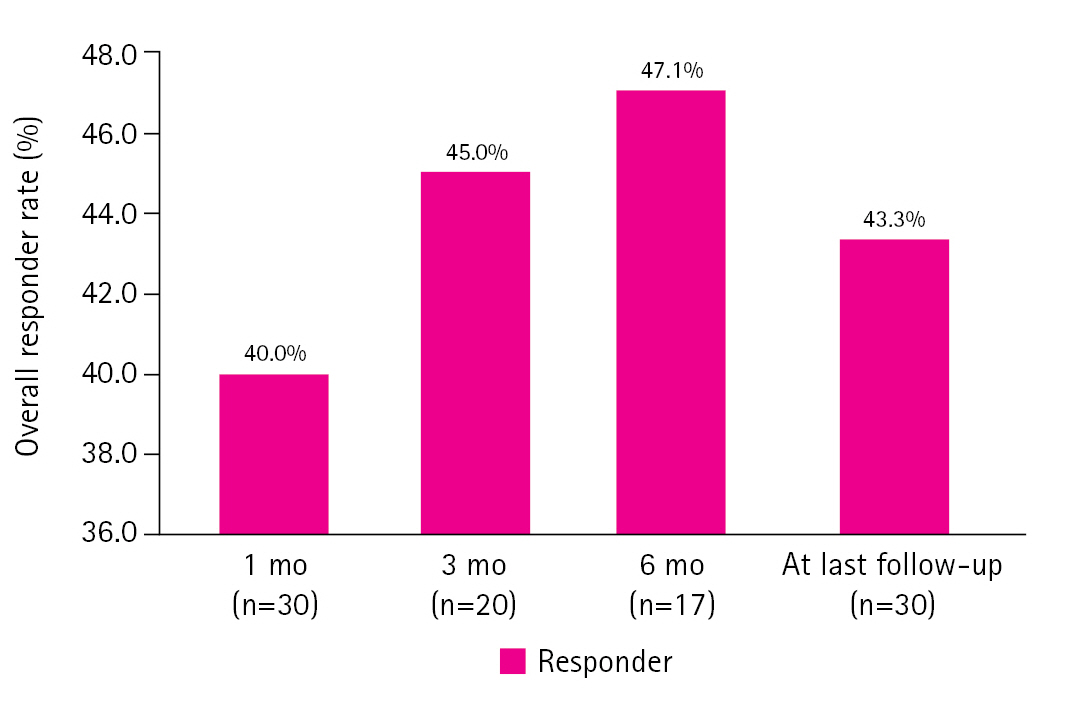
During the treatment period, 16 patients showed ≥50% seizure reduction without serious adverse events. The seizure outcomes of the study group at 1, 3, and 6 months and at the last follow-up are shown in Fig. 1. The response rate (i.e., the proportion of patients who achieved ≥50% monthly seizure frequency reduction) was 40% at 1 month, 45% at 3 months, 47.1% at 6 months, and 43.3% at the last follow-up (Fig. 1).
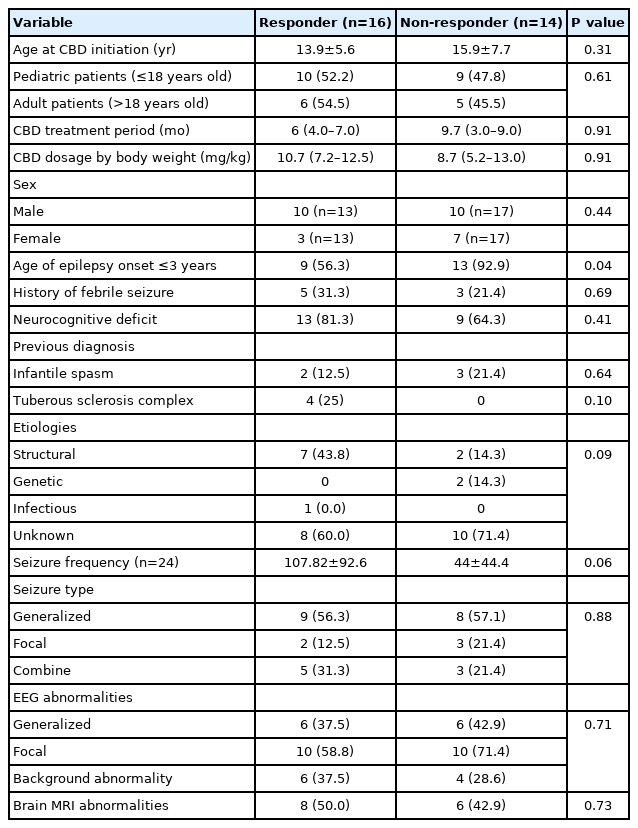
The study group was divided into two subgroups according to seizure outcomes at the last follow-up (responders [n=16] vs. non-responders [n=14]). When comparing the two subgroups, demographic findings, past medical history, epilepsy etiologies, and the type of seizure or EEG findings were not associated with response to CBD. However, there was a difference in the proportion of patients who were diagnosed with epilepsy at an older age (>3 years of age), which suggests that those patients tended to respond better to CBD treatment (Table 2). Furthermore, all four patients with TSC were included in the responder group. In the responder group, there was a meaningful monthly seizure frequency reduction by seizure type (generalized seizure [n=14, 66.6±76.9 vs. 16.7±14.1, P=0.02] vs. focal seizure [n=7, 134.1±121.9 vs. 41.2±50.8, P=0.04]) (Fig. 2). In the univariate analysis, patients whose epilepsy onset occurred after 3 years of age responded better to CBD (odds ratio [OR] for response, 10.11; 95% confidence interval [CI], 1.05 to 97.00; P=0.045) (Table 3).
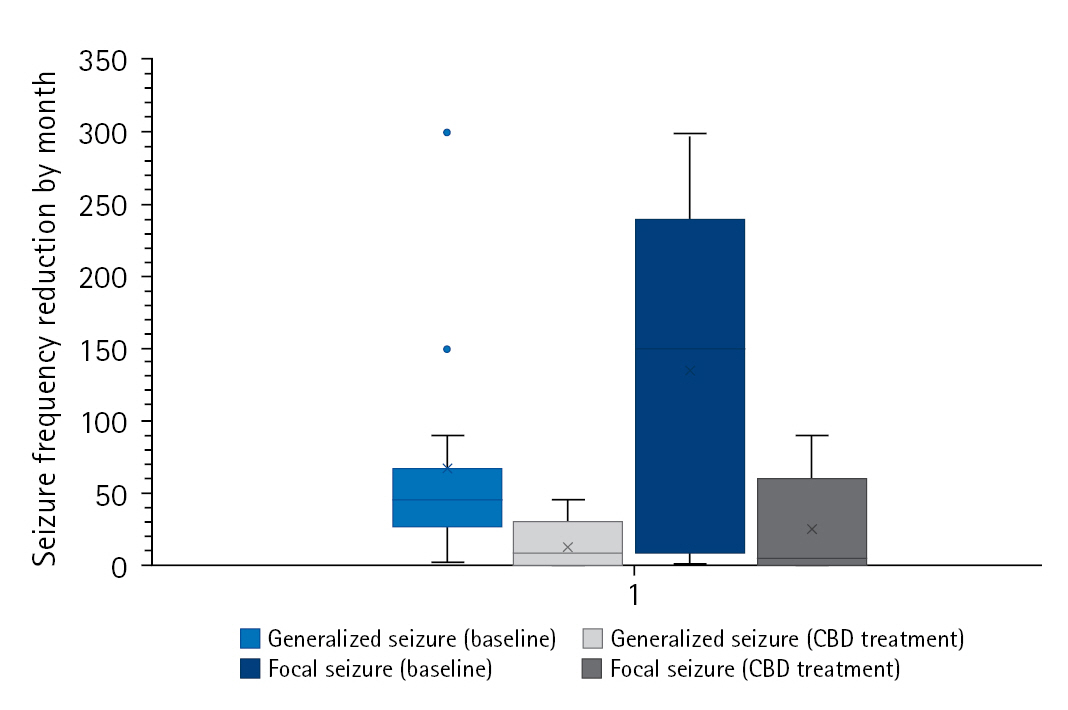
Monthly mean seizure frequency reduction at baseline and the last follow-up in the responder group. CBD, cannabidiol.
When compared by age group, adult (11 patients) and pediatric (aged ≤18 years, 19 patients) subgroups showed no significant differences in the likelihood of achieving ≥50% seizure reduction (6/11 [54.5%] vs. 10/19 [52.2%]) or in the adverse event rate (4/11 [36.4%] vs. 7/19 [36.8%]).
Subjective reports from patients’ caregivers described improved neurocognitive function in 6/30 (20%) patients, and 7/30 (23.3%) patients de-escalated their ASMs during the follow-up period after CBD introduction.
During the treatment period of our study, 12 (40%) patients discontinued their treatment for various reasons. Five patients complained about the lack of efficacy of CBD treatment, another four experienced unexpected adverse events (somnolence, n=2; ataxia, n=1; and diarrhea, n=1), one discontinued the medication due to an economic burden, one could not visit the clinic due to the coronavirus disease 2019 outbreak, and one wanted to escalate other ASMs.
Discussion
This retrospective study analyzed data from real-world practice in South Korea and revealed comparable results for the overall response rate (16/30, 53.3%) and the adverse event rate (11/30, 36.7%). There was a meaningful monthly seizure frequency reduction in the generalized and focal seizure frequencies in the responder group. In the univariate logistic analysis, age at epilepsy onset (>3 years) was associated with a good response to CBD during the treatment period.
In two randomized controlled studies conducted in 2018 on the response to medical cannabis treatment for LGS, it was discovered that cannabinoids showed significant monthly seizure frequency reduction in LGS patients, especially CBD, which was effective for drop seizures [5,10]. Further open-label extended studies proved that long-term add-on CBD therapy sustained total and drop seizure reduction in LGS [11], and higher doses as an adjunctive treatment (up to 50 mg/kg/day) showed significant monthly seizure frequency reduction for mixed seizure etiologies [12]. In another study on effective dose ranges for convulsive seizures in DS, adjunctive CBD led to reductions in convulsive seizure frequency, with a better safety and tolerability profile for the 10 mg/kg/day dose [13]. Another study group in South Korea reported CBD’s effects on patients with LGS and DS, stating that substantial proportions of patients experienced a ≥50% seizure frequency reduction at 3-month (LGS 32.3% and DS 30%) and 6-month (LGS 29.4% and DS 20%) follow-ups, at a mean dosage of 10 mg/kg/day [14]. In our study, 16 (53.3%) patients achieved ≥50% seizure reduction, with a median dosage of 10.7 mg/kg/day (IQR, 7.2 to 12.5). A meaningful difference was found in monthly seizure frequency reduction by seizure type (generalized seizure vs. focal seizure: 75% vs. 70%). As serious adverse events such as liver transaminase elevation, lethargy, sedation, and upper respiratory tract infection occurred in a dose-dependent manner, our results support the notion that low-dose CBD is effective, with tolerable safety, for focal and generalized seizure frequency reduction in patients with LGS.
Evidence of CBD treatment for patients with TSC has also been widely reported. Hess et al. [15] studied CBD for drug-resistant TSC and proved that the median weekly seizure frequency decreased from 22.0 (IQR, 14.8 to 57.4) to 13.3 (IQR, 5.1 to 22.1) after 3 months of treatment with CBD. In a randomized controlled trial conducted in 2019, there was a significant monthly seizure frequency reduction from baseline to 48.6% (95% CI, 40.4% to 55.8%) for the CBD25 (25 mg/kg/day) group and 47.5% (95% CI, 39.0% to 54.8%) for the CBD50 (50 mg/kg/day) group [7]. An extended open-label study also proved stable efficacy (median percentage reduction of 54% in TSC-associated seizures) and improved overall conditions reported by caregivers [10]. Our study included four patients with TSC, and all four responded to a mean effective dose of CBD of 13.14 mg/kg/day. However, as our study population included a small number of patients with TSC, further well-designed and large-scale analyses are required.
One of our key findings was that the patients responded better to CBD treatment when epilepsy onset occurred at an age >3 years (OR, 10.11; 95% CI, 1.05 to 97.00; P=0.045). Several previous studies have suggested that the outcome of epilepsy with an earlier onset (in the first few years of life) has a worse prognosis than that with a later onset [16,17]. An earlier onset of epilepsy is caused by structural abnormalities in the brain, such as cerebral malformations, neurometabolic diseases, and perinatal hypoxic-ischemic encephalopathy, which are significantly associated with treatment response to ASMs [18]. Thus, the better response to CBD in patients with epilepsy onset after 3 years of age may be explained by the fact that an earlier onset of epilepsy is associated with abnormalities of the brain such as perinatal structural brain abnormalities and electroclinical syndrome, which are already known risk factors for intractable seizures. However, in order to clarify which clinical factors were associated with response to CBD, a more detailed study design investigating etiology and other clinical factors is required.
Positive effects on neuro-cognition were observed in six (20%) patients treated with CBD. There is growing evidence for the improvement of neurocognition and sleep architecture after treatment with CBD [10,15,19-21]. Due to the nature of real-world study data with a small population and the nature of LGS, which is usually accompanied by intellectual disabilities, the neuro-cognitive improvements in our study could not be objectively evaluated using a neuropsychiatric assessment tool, and the main resources for the cognitive and behavioral improvements were caregivers’ subjective documentation. Further well-designed studies with well-documented records are required.
The main limitations of this study are its retrospective design and small sample size. We did not review specific types of ASMs. Since the mechanism of action of medical cannabis has not yet been fully elucidated and CBD is considered to be a potent inhibitor of certain hepatic enzymes, there could be possible drug-drug interactions that may influence the overall effect of CBD. As we did not include patients treated with CBD for less than 1 month, the tolerability and safety of CBD were underestimated. The types of seizures were mainly based on the reports of the patients’ caregivers, and efficacy for specific types of seizures, such as atonic, myoclonic, and drop seizures, could not be evaluated. Moreover, the duration of the treatment period was insufficient due to the recent introduction of CBD in South Korea and delayed permission for national health insurance coverage. A further prospective study with a large population and longer-term follow-up is needed to evaluate the tolerability and efficacy of CBD in patients with types of drug-resistant epilepsy other than LGS.
CBD could be a treatment option for children and young adults with drug-resistant LGS with a tolerable safety profile. Age at epilepsy onset (>3 years) was associated with a favorable response to CBD treatment. Further prospective studies with a large population are needed to evaluate the tolerability and efficacy of CBD in patients with drug-resistant epilepsy of varying etiologies.
Notes
Tae-Sung Ko is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: HNJ, MJK, MSY, and TSK. Data curation: HNJ, MJK, and MSY. Formal analysis: TSK. Funding acquisition: MSY and TSK. Methodology: HNJ, MJK, MSY, and TSK. Project administration: MSY. Visualization: HNJ. Writing-original draft: HNJ. Writing-review & editing: HNJ, MSY, and TSK.