Gamma-Knife Radiosurgery for Hypothalamic Hamartoma-Related Epilepsy
Article information
Abstract
Purpose
Hypothalamic hamartoma (HH), a rare congenital disorder, can cause intractable epilepsy and requires optimal surgical treatment. This study analyzed the clinical characteristics of HH and evaluated seizure outcomes and the safety of gamma-knife radiosurgery (GKS) in HH-related epilepsy to propose an optimal surgical treatment.
Methods
We reviewed the medical records of 18 patients with HH treated at Samsung Medical Center (1997 to 2018), and analyzed their presenting symptoms, brain magnetic resonance imaging (MRI) findings, treatment, and response.
Results
The median diagnostic age was 3.2 years. The first presenting symptom was a seizure in six (33.3%), precocious puberty in five (27.8%), both symptoms in six (33.3%), and no symptoms in one patient who was diagnosed incidentally on brain MRI. All mixed and intrahypothalamic types except one had seizures (n=12), while all five parahypothalamic types presented only precocious puberty. Eleven patients showed intractable epilepsy with medications and underwent surgical treatment (most commonly GKS). Eight patients underwent GKS, and two of them received repeated GKS for recurrent seizures. Six patients showed improved seizure control with GKS as the last treatment. Among them, two patients became seizure-free, and one patient had a decreased frequency of seizures after a single GKS. There was no adverse effect related to GKS.
Conclusion
Intractable epilepsy was the most common indication for surgical treatment of pediatric HH. GKS was effective in controlling seizures in 75% of HH patients without any adverse effects. Repeated GKS could also be considered as a safe option for intractable and disabling seizures.
Introduction
Hypothalamic hamartoma (HH) is a rare congenital malformation of the ventral hypothalamus. The estimated incidence of HH ranges from 1 in 50,000 to 1 in 100,000, and that of HH with epilepsy ranges from 1 in 200,000 to 1 in 625,000, according to a population-based study [1]. HH mainly presents with seizures, typically gelastic seizure (GS), and central precocious puberty. Clinical symptoms are also known to be associated with the location. HH connecting to the posterior hypothalamus in the region of the mammillary bodies is associated with epilepsy [2], while HH in the anterior hypothalamus and in the region of the tuber cinereum is associated with central precocious puberty [3]. Furthermore, neurobehavioral comorbidities, such as cognitive impairment and psychiatric symptoms, are frequently present in patients with HH [4-6].
Numerous studies have established the cellular and structural mechanisms of epileptogenic pathogenesis in HH. Implanted intracranial electrodes in HH lesions record seizures associated with HH [2]. HH shows normal cell shapes; however, the cells have an abnormal organization, with variable neuronal size and density, and present pacemaker-like firing activity or excitatory projection-type neuronal phenotypes [7]. Therefore, a surgical treatment that disconnects the abnormal pacemaker from the normal brain tissue is considered in cases of HH with intractable epilepsy.
As HH with epilepsy usually tends to be intractable to anti-seizure medication (ASM), surgical approaches, including resection or ablation of the hamartoma, may be a suitable treatment option. However, the location and anatomical relationship of the HH limit surgical approaches, and the options vary depending on the patient’s age. Surgical treatments for HH are mainly divided into the following: invasive methods, such as lesion resection with open craniotomy; minimally invasive methods, such as laser interstitial thermal therapy (LiTT) [8], stereotactic thermocoagulation (stereotactic radiofrequency thermocoagulation [SRT], or radiofrequency ablation [RFA]); and noninvasive methods, such as gamma-knife radiosurgery (GKS) [9,10]. Although direct lesionectomy had better surgical outcomes than other methods, it showed a high complication rate and had restrictions on accessing the lesion [11]. Minimally invasive and noninvasive methods have been suggested to reduce these limitations, but there are also considerations such as patient’s age and size of HH [12,13].
Despite advances in surgical techniques and outcomes [14], there are difficulties in studying HH, such as the low incidence rate, variable prognosis according to HH location and size, and different experiential guidelines across various countries [15]. Therefore, further HH research is needed to suggest a guideline for surgical treatment and prognosis. Here, we delineated the clinical manifestation of HH and treatment of HH-related epilepsy and presented the effects and advantages of GKS as a preferred surgical treatment.
Materials and Methods
This study included pediatric patients who were diagnosed with HH between 1997 and 2018 at Samsung Medical Center. We retrospectively reviewed their medical records and analyzed the clinical manifestations, brain magnetic resonance imaging (MRI) findings, treatments for each symptom, and responses to those treatments. We classified the HH type according to the classification of Kameyama et al. [16]. The intrahypothalamic type was defined as being located in the third ventricle, while the parahypothalamic type extended inferiorly to the interpeduncular fossa. The mixed hypothalamic type included components of both the intrahypothalamic and parahypothalamic types. Furthermore, we analyzed treatment response based on both Engel's classification and the International League Against Epilepsy (ILAE) [17].
We examined the clinical manifestations of precocious puberty, including breast budding, vaginal bleeding, increased testis volume, and pubic hair in the pediatric clinic and confirmed precocious puberty through laboratory tests of sex hormone (basal and stimulation tests) in patients with clinical suspicion.
1. Ethics statement
This study was approved by the Institutional Review Board of the Samsung Seoul Hospital (IRB File No. 2019-10-036). Informed consent was waived by the board.
Results
1. Clinical manifestations
Eighteen patients were diagnosed with HH at a median age of 3.2 years (range, 0.08 to 10.5) and were followed up for a median of 7.35 years (range, 0.4 to 18.6). The presenting clinical manifestation was a seizure in 12 patients (66.7%) and precocious puberty in 11 patients (61.1%). Six patients (33.3%) presented with both seizure and precocious puberty, and one patient was incidentally diagnosed with HH during the evaluation of an arachnoid cyst that had been detected on fetal sonography (Supplementary Fig. 1).
The most common type of seizure was GS, which was observed in 11 patients (91.7%), while one patient (8.3%) presented with focal seizures without GS. Seizures presented at a median age of 1.58 years (range, 0.08 to 6), and precocious puberty was diagnosed at a median age of 5 years (range, 0.67 to 10.5) (Table 1). We conducted neuropsychological tests only for patients who were suspected of having an intellectual disability or other psychological symptoms. Four patients (patients 2, 3, 7, and 9) underwent testing, and all were revealed to have mild-to-moderate intellectual disability. Additionally, patients 3 and 9 were diagnosed with attention deficit hyperactivity disorder and paranoid personality disorder, respectively (Table 1).
1) Relationship between clinical symptoms and radiological classification
Three patients (16.7%) were classified as having intrahypothalamic HH, five patients (27.8%) as having parahypothalamic HH, and 10 patients (55.6%) as having mixed hypothalamic HH. One patient with the intrahypothalamic type presented with seizures only and without precocious puberty, while none of the patients with the parahypothalamic type experienced seizures. All patients with the mixed hypothalamic type experienced seizures, and six (60%) had both seizures and precocious puberty. Patient 8, who was incidentally diagnosed via post-natal brain MRI for a large arachnoid cyst, has the parahypothalamic type and did not demonstrate any symptoms until the age of 14 years. Fig. 1 shows the brain MRIs at the initial diagnosis at 1 month of age (Fig. 1A) and follow-up at 2 months (Fig. 1B) and 9 years (Fig. 1C) after endoscopic fenestration.
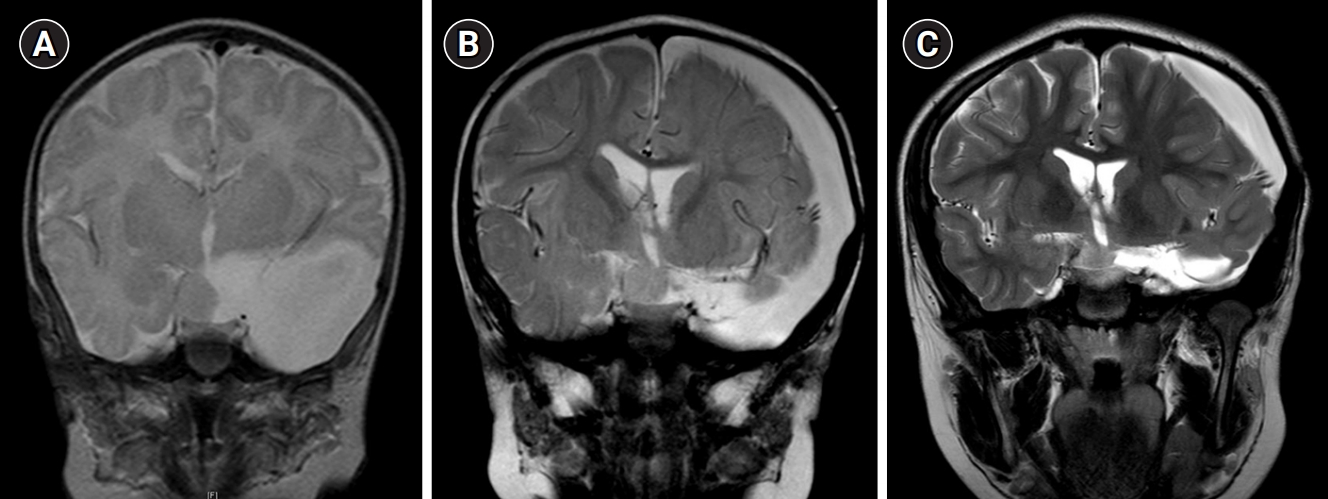
Coronal T2-weighted brain magnetic resonance imaging (MRI) findings of an asymptomatic patient. Patient 8 was incidentally diagnosed via brain MRI after birth for the evaluation of a large arachnoid cyst found on fetal ultrasonography. The patient underwent penetration surgery for the large arachnoid cyst at the age of 9 months. (A) Initial brain MRI at the age of 1 month; (B) brain MRI at the age of 11 months (2 months after endoscopic penetration surgery); (C) brain MRI at the age of 10 years (9 years after panel B).
2. Treatment modalities of intractable epilepsy
Among 18 patients with HH, 12 patients initially presented with seizures. Three patients were not followed up after presenting with a seizure, patient 16 was seizure-free for 3 months on a single ASM, and patients 13 and 14 were scheduled to undergo SRT at another hospital but were lost to follow-up (Fig. 2). The remaining nine patients did not respond to multiple ASMs (median, 2; range, 0 to 4) over a median period of 0.17 years (range, 0 to 3.25) (Table 2). Eight patients underwent GKS, and one patient (patient 17) underwent only SRT at another hospital in Japan.

Surgical treatments of 12 patients with seizures (sz). This flow chart demonstrates the treatment course based on seizures. The bar graph indicates the patients with each type of hypothalamic hamartoma; intrahypothalamic (I), parahypothalamic (P), and mixed (M) types are shown as striped, gray, and black columns, respectively. SRT, stereotactic radiofrequency thermocoagulation; ASM, anti-seizure medication; GKS, gamma-knife radiosurgery; STR, subtotal resection; GS, gelastic seizure; FU, follow-up; ED, endoscopic disconnection; RFA, radiofrequency ablation.

Radiologic characteristics and seizure outcomes in patients who underwent surgery for hypothalamic hamartoma
Eight patients underwent GKS at a median age of 6.7 years (range, 4.2 to 13.2), which was based on the initial treatment of GKS. After the initial GKS, patients 2 and 4 could discontinue ASMs and became seizure-free for 11 and 4 years, respectively. Patient 9 demonstrated improved seizure control for 3 years, with decreased ASMs (Table 2). Patient 1, who had undergone subtotal resection at the age of 18 months, underwent GKS at the age of 13 years. She no longer had focal and generalized seizures after GKS. However, her GS did not decrease significantly despite the GKS, and she continued with ASMs during the 6 years until her last follow-up.
Four patients (patients 3, 7, 11, and 12) showed no improvement in seizure frequency after the initial GKS (last step of Fig. 2). Patient 7 underwent endoscopic disconnection (ED) after GKS, and his GS was reduced by over 50%; however, generalized tonic and focal seizures with impaired awareness newly appeared. Patient 12 had no improvement in GS frequency and recently underwent SRT at another hospital 2 years after the initial GKS.
1) Repeated GKS
Two patients (patients 3 and 11) underwent repeated GKS after the first GKS. They presented no deleterious adverse effects and had a 50% or more reduction in seizure frequency. Patient 11, who had uncontrolled seizures after the first GKS, was treated with a second GKS, became seizure-free for 2 years until the last follow-up, and was able to reduce the number of ASMs from 3 to 2. Patient 3 underwent GKS at the age of 6.5 years and had no seizures for 3 years. He showed recurrence of seizures at the age of 8.6 years and underwent RFA as the second surgical option (Table 2). He had a seizure-free period for 2 years with ASM after RFA; however, he had seizures again. He was then treated with a second GKS, following which a seizure-free state was maintained for 1 year. Fig. 3 shows the brain MRI of two patients with repeated GKS before and after surgery.

Coronal T2-weighted brain magnetic resonance imaging (MRI) of two patients who underwent repeated gamma-knife radiosurgery (GKS). Brain MRI of patient 11 was obtained (A) at the age of 4 months, (B) at the age of 6 years 10 months (15 months after the first GKS), and (C) at the age of 10 years (14 months after the second GKS). Brain MRI of patient 3, who underwent radiofrequency ablation (RFA) between the first and second GKS (a second GKS 2 years after undergoing RFA), was obtained (D) at the age 6 years, (E) at the age of 15 years (8 years after the first GKS), and (F) at the age of 16 years (1 year after RFA and 1 year before the second GKS).
3. Overall surgical outcome in epilepsy treatment
Based on Engel’s classification, four of the eight patients who underwent GKS were categorized as IA and two patients as ID after GKS as the last treatment. Based on the ILAE, four of the eight patients with GKS were categorized as class 1 and two patients as class 4 after GKS as the last treatment. Patient 12 showed a seizure outcome of IB/class 4, and the last surgical treatment was SRT. Patient 7, who underwent ED after GKS, was classified as IVC and class 6 (Table 2). Patient 17, who had a single SRT, was classified as IB and class 4.
Discussion
This study evaluated the surgical options and treatment results of 18 pediatric patients with HH. Seizure, which is an early predominant symptom, was intractable to medical treatment in 91.7% of the patients, and this was the leading cause of surgical treatment. In this study, GKS was the most common surgical treatment, and eight patients underwent GKS. Among these patients, six (n=6/8) became seizure-free or had a >50% reduction in seizure frequency, without any significant complications. Two patients underwent GKS twice and showed good seizure control without any complications associated with the procedure.
Epilepsy in patients with HH is mostly intractable to medical treatment [18,19]. In our study, 11 of 12 patients (91.7%) had uncontrolled seizures on ASMs, except for one patient (patient 16) who had been followed up for only 4 months. All of these patients underwent surgical treatment, and 88.9% showed controlled seizures. Therefore, surgical resection or disconnection has been considered a promising cure for HH-associated epilepsy [20]. In the past, classical resection with craniotomy was performed, but due to the risks related to open surgery, it has been replaced by less invasive methods, including the transcallosal approach, brachytherapy, ED, LiTT, RFA, and GKS [21-30]. Table 3 shows seizure outcomes and adverse effects of these surgical approaches, all of which have shown as favorable outcomes as direct lesion resection [8,12,31-35]. If all of the various surgical methods have good seizure outcomes, it is reasonable to choose a method that is relatively less invasive and has fewer adverse effects. In that sense, GKS can be one of the attractive options. GKS presents favorable outcomes for seizure control and several studies reported 60% to 68% improvement in seizure control, with no major adverse effects [30,33-35]. In our study, seven patients underwent GKS as the initial surgical treatment. Among them, three patients (patients 2, 4, and 9; 42.9%) had effective seizure control, with Engel class IA, after a single GKS. Another four patients had additional surgical treatments, such as RFA, second GKS, ED, or SRT, because of persistent seizures after the initial GKS [33].
GKS has the lowest incidence of endocrine dysfunction compared to other methods, because it delivers minimal radiation to the targeted tissue [3]. In our study, there were no reported endocrine adverse effects directly associated with GKS. Another advantage of GKS is that its safety is high even when performed multiple times. Second GKS was performed in 58.3% of patients in a previous prospective study [33]. Two of our patients (patients 3 and 11) underwent a second GKS, and there were no remarkable adverse effects. In a previous study consisting of 137 patients (median age, 27.1 years; range, 0.6 to 84.8) with craniopharyngioma, tumor control was better in 25 patients who underwent repeated GKS. Only 10.9% of all patients showed GKS-related complications, including hypopituitarism (8.0%), adverse radiation effects (1.5%), visual deterioration (1.5%), and newly developed cranial nerve palsy (0.7%) [36]. Considering the low adverse effect profile, repeating GKS would be considered for patients who show a partial response to the first GKS to improve the treatment outcome.
GKS has some limitations. It is not recommended for large HH due to biophysical deterioration of the high radiation dose to surrounding tissues in such cases, and other surgical treatments need to be considered for giant HH (>30 mm) [12]. Additionally, the delayed therapeutic effect supports the application of other treatment choices, given that treatment timing might prevent other clinical manifestations of HH. Age can be another obstacle for applying GKS because the skull in younger patients is too brittle for the frame to be applied. More precise stereotactic techniques and suitable frames could help to overcome the limitations of GKS treatment in HH patients.
In conclusion, seizures in HH are mostly intractable and constitute a major motivation for surgical treatment. There are many surgical options for HH, for each of which several factors should be considered, including the degree of invasiveness, associated adverse effects, and availability. This study demonstrated a favorable overall outcome of GKS without associated adverse effects in controlling HH-related seizures. Considering its safety and comparable efficacy, GKS can be considered as the preferential surgical treatment option for HH patients with intractable epilepsy. In addition, regarding its delayed effect, GKS can be chosen in cases with a poor response after other surgical treatments. Moreover, repeated GKS could be considered if the first GKS shows a partial response. For optimal guidance for surgery in patients with HH, further research with more cases from multiple centers and systematic evaluations is needed.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.26815/acn.2021.00360.
Clinical manifestation and radiologic diagnosis. The black square on the left indicates the patients who presented with seizures and that on the right represents the patients who presented with precocious puberty. Each column in the graph shows the distribution of the type of hypothalamic hamartoma based on the clinical manifestation; stripe, gray, and black columns represent intrahypothalamic (I), parahypothalamic (P), and mixed type of hypothalamic hamartoma (M), respectively.
Notes
Jeehun Lee is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: JL and JL. Data curation: JYS and JL. Formal analysis: JYS. Methodology: JIL, HJS, JL, and JL. Project administration: JL and JL. Visualization: JYS. Writing-original draft: JYS. Writing-review & editing: JL and JL.
Acknowledgements
We appreciate the participation and cooperation of the patients and their families during the study.


