The Efficacy and Safety of Rituximab for the Treatment of Pediatric Autoimmune Neuroinflammatory Disorders at a Single Center
Article information
Abstract
Purpose
Rituximab is increasingly used as a second-line treatment of neuroinflammatory disorders to improve clinical outcomes in cases refractory to conventional immunotherapy and to reduce relapses. This study aimed to demonstrate the efficacy and safety of rituximab used for pediatric autoimmune neuroinflammatory disorders.
Methods
We retrospectively reviewed the medical records of 32 patients (median age, 8.5 years; range, 1.1 to 17.1; 23 girls) who received rituximab treatment at Seoul National University Children’s Hospital. The disease subgroups were anti-N-methyl-D-aspartate (NMDA) receptor (anti-NMDAR) encephalitis (n=11), opsoclonus-myoclonus ataxia syndrome (OMAS) (n=10), other suspected autoimmune encephalitis (n=5), neuromyelitis optica spectrum disorder (n=4), and chronic inflammatory demyelinating polyneuropathy (n=2). Efficacy was measured by modified Rankin Scale (mRS) scores at the initiation of rituximab administration, at 2 months after initiation, and at the last follow-up. A favorable clinical outcome was defined as an improvement of ≥2 in the mRS score or achievement of an mRS score ≤2. Safety was assessed by reviewing infusion-related adverse events and infectious complications, including progressive multifocal leukoencephalopathy.
Results
Two months after the initiation of rituximab therapy, 21patients (65.6%) had a favorable response, while 26 (82.1%) had a favorable response at the last follow-up. Among the disease subgroups, anti-NMDAR encephalitis and OMAS showed especially good responses. Rituximab infusion-related adverse events were identified in nine patients (28.1%). All complications recovered spontaneously or with only symptomatic treatment.
Conclusion
Rituximab can be used safely for various pediatric autoimmune neuroinflammatory diseases. Rituximab is expected to improve clinical outcomes in pediatric patients with anti-NMDAR encephalitis and OMAS.
Introduction
Rituximab is a chimeric monoclonal anti-CD20 antibody that induces B-cell depletion, resulting in a decrease in antibody-mediated immunity [1]. Since it was approved for treatment of non-Hodgkin’s B-cell lymphoma, the indications for use of rituximab have expanded to include diverse autoimmune disorders including rheumatoid arthritis, systemic lupus erythematosus, and nephrotic syndrome [2-5]. In neurology, the use of rituximab in a number of autoimmune neuroinflammatory disorders has increased significantly, including in multiple sclerosis, anti-N-methyl-D-aspartate (NMDA) receptor (anti-NMDAR) encephalitis, opsoclonus-myoclonus ataxia syndrome (OMAS), neuromyelitis optica spectrum disorder (NMOSD), myasthenia gravis (MG), and chronic inflammatory demyelinating polyneuropathy (CIDP) [6-12]. The purposes of rituximab use are either to induce short-term remission in refractory disorders (e.g., NMDAR encephalitis and OMAS) or to prevent the occurrence of relapses (e.g., NMOSD, MG, and CIDP) [8,9,13]. While there is accumulating evidence of the efficacy of rituximab, safety data, especially in pediatric patients, have been relatively scarce, except for one large cohort study, which reported a variety of adverse events related to rituximab including fever, chills, skin rash, headache, tachycardia, anemia, thrombocytopenia, and neutropenia [14]. We performed the present study to investigate the efficacy and safety of rituximab for pediatric autoimmune neuroinflammatory disorders.
Materials and Methods
1. Study population
We collected a list of patients who received rituximab treatment at Seoul National University Children’s Hospital between January 1, 2009 and December 31, 2018. From these 104 patients treated with rituximab, we selected 32 patients with autoimmune neuroinflammatory disorders including anti-NMDAR encephalitis, OMAS, other suspected autoimmune encephalitis, NMOSD, and CIDP. The definition of each subgroup is provided in Supplementary Table 1 [15-18]. This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB no. H-1911-201-1086). Written informed consents from patients were waived due to a retrospective nature of the study.
2. Analysis of clinical variables and laboratory data
Clinical variables (demographic data, age at onset, diagnosis, first-line treatment, age at rituximab administration, and intensive care unit admission) were collected from the medical records. The complete blood count was analyzed to evaluate hematologic adverse events.
3. Rituximab treatment protocol
All patients received first-line treatment including corticosteroid, intravenous immunoglobulin (IVIG), or plasmapheresis. The dose and treatment duration of each first-line immunotherapy are listed in Supplementary Table 2. Rituximab was administered to patients who showed a refractory (NMDAR encephalitis, OMAS, and other suspected encephalitis) or recurrent clinical course (NMOSD and CIDP) after first-line immunotherapy. The treatment protocol consisted of 4 weekly rituximab infusions over 1 month. The dose of rituximab at each infusion was 375 mg/m2 (maximum 500 mg). All patients received prophylactic medications 30 minutes before rituximab infusion: oral acetaminophen (10 mg/kg), intravenous pheniramine (0.1 mg/kg, maximum 4 mg), and intravenous hydrocortisone (4 mg/kg, maximum 100 mg). The CD19+ cell count was measured before and after the four rituximab infusions. The decision whether to add additional rituximab infusion after one cycle of treatment was made on a clinical basis.
4. Analysis of efficacy outcome and safety
Clinical status was assessed using modified Rankin Scale (mRS) scores at the initiation of rituximab therapy and at last follow-up. Favorable clinical response parameters were defined as achievement of a mRS score ≤2 points, or an improvement of ≥2 points in mRS score at 2 months after initiation of rituximab and at the last follow-up [8,14,19,20].
Acute infusion-related adverse events were defined as unexpected and unfavorable responses that developed during the infusion of rituximab. Infectious adverse events were defined as complications that were related to immunosuppression after rituximab infusion. All management of adverse reactions was recorded. All adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE v. 5.0) [14].
Results
A total of 32 patients were included in the analysis. The median age at disease onset was 8.5 years (range, 1.1 to 17.1). The duration of follow-up after rituximab administration was a median 2.1 years (range, 0.2 to 8.1).
The demographic data of the patients and their disease subgroups are summarized in Table 1. First-line treatment with methylprednisolone was administered to 34 (97.1%), IVIG to 32 (91.4%), and plasmapheresis to seven patients (20%).
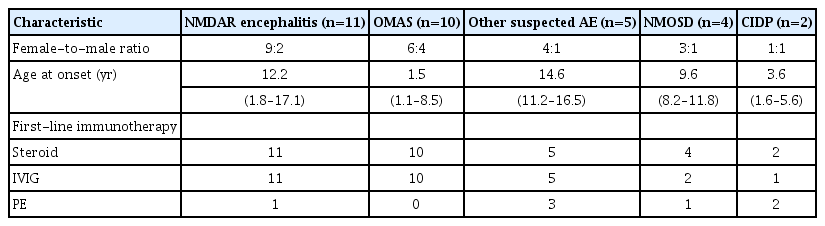
Demographic characteristics, age at disease onset, and first-line treatment according to disease subgroup
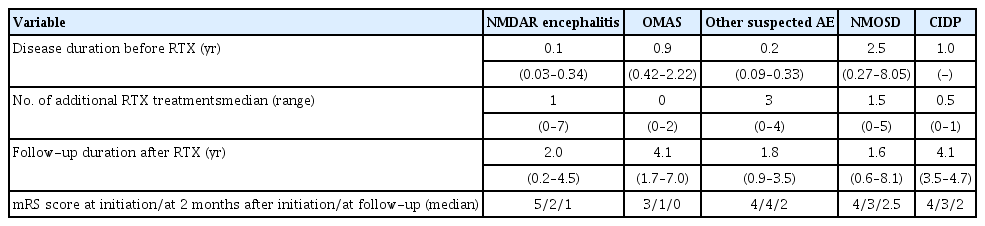
The median age at initiation of rituximab therapy was 9.0 years (range, 1.6 to 19.8) and the median duration of disease before rituximab therapy was 0.3 years (range, 0.03 to 8.05). The median number of additional rituximab infusions after the initial 4 weekly infusions was 1 (range, 0 to 7). The variety of the time to rituximab therapy and the number of additional therapy after one cycle according to disease subgroup is presented in Table 2.
1. Efficacy outcome
Five of 32 patients (15.6%) were admitted to the intensive care unit at initiation of rituximab therapy. The median mRS scores were 4 (range, 2 to 5) at initiation of rituximab therapy, 2 (range, 1 to 5) at 2 months after initiation of therapy, and 1 (range, 0 to 5) at last follow-up. Twenty-one patients (65.6%) showed a favorable outcome at 2 months after initiation and 26 patients (82.1%) at last follow-up. The mRS scores according to disease subgroup at initiation of rituximab therapy, at 2 months after initiation, and at last follow-up (average duration, 3.0±2.0 years) are presented in Fig. 1.
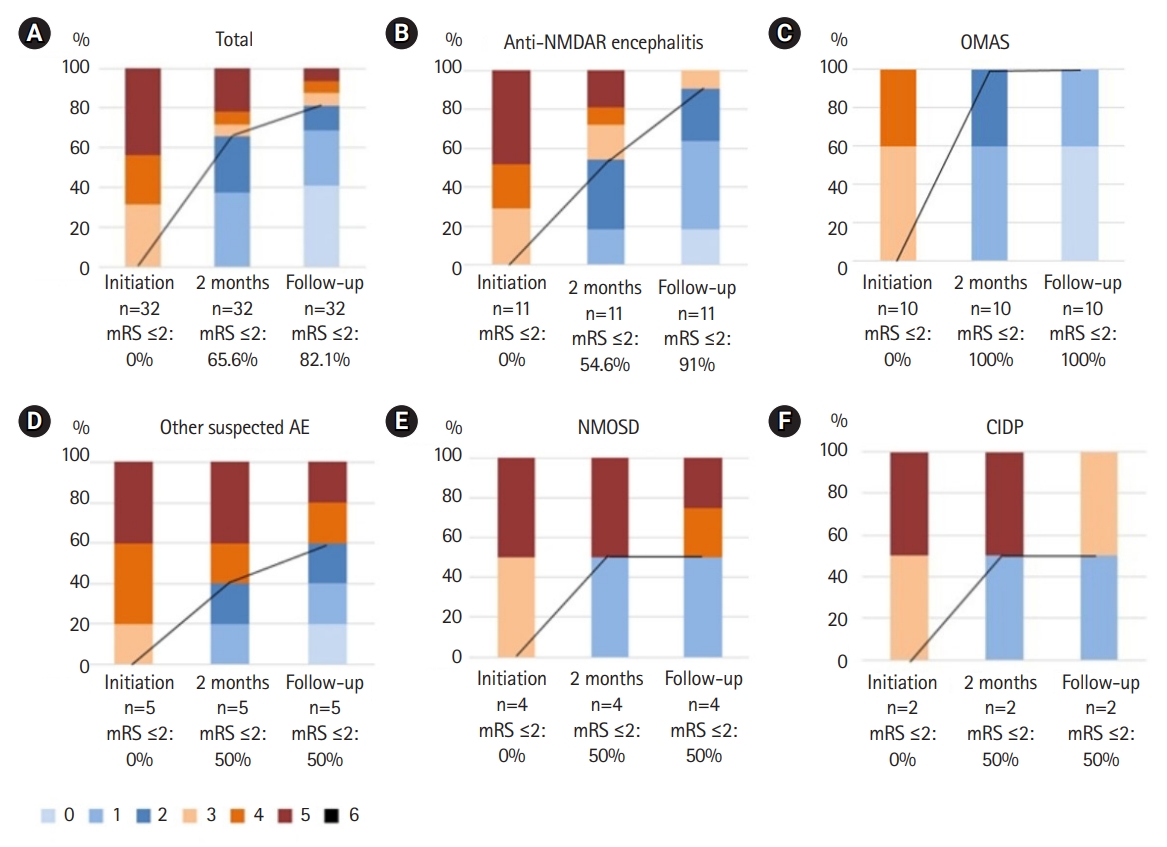
Modified Rankin Scale (mRS) score at the initiation of rituximab, 2 months after the initiation of rituximab, and the last follow-up. Comparison of mRS scores for all patients (total) and subgroups. The groups are as follows: (A) total (n=32); (B) anti-N-methyl D-acetyl receptor (NMDAR) encephalitis group (n=11); (C) opsoclonus-myoclonus ataxia syndrome (OMAS) group (n=10); (D) other suspected autoimmune encephalitis (AE) group (n=5); (E) neuromyelitis optica spectrum disorder (NMOSD) group (n=4); (F) chronic inflammatory demyelinating polyneuropathy (CIDP) group (n=2). The black line represents the change in the proportion of patients with mRS scores ≤2 at rituximab initiation, 2 months after the initiation of rituximab, and the last follow-up.
The improvement in outcome evaluated by mRS scores is presented above with the clinically compatible rate for all patients and for each disease subgroup. Among the disease subgroups, anti-NMDAR encephalitis and OMAS showed especially favorable responses. In the anti-NMDAR encephalitis group, the median mRS score was 5 (range, 4 to 5) at initiation, 2 (range, 1 to 5) at 2 months, and 1 (range, 0 to 3) at last follow-up. A favorable outcome was demonstrated in six patients (54.6%) at 2 months and 10 patients (91%) at last follow-up. In the OMAS group, the median mRS score was 3 (range, 3 to 4) at initiation, 1 (range, 1 to 2) at 2 months, and 0 (range, 0 to 1) at last follow-up. A favorable outcome was demonstrated in all 10 OMAS patients (100%) at 2 months and at last follow-up. NMOSD patients showed not only an improvement in neurological function, but also a decrease in the annual relapse rate from 2.5 per year (range, 2 to 3) to 0.5 per year (range, 0 to 1). Two patients with anti-NMDAR encephalitis and three patients with other suspected autoimmune encephalitis showed refractory clinical courses despite additional rituximab therapy, and they received other immunotherapies including cyclophosphamide and tocilizumab (data not shown).
All 32 patients showed the decrease of B-cell count after one cycle of rituximab. Before rituximab treatment, the median CD19+ B-cell count was 617/μL (range, 45 to 2,213), and the median proportion of total CD19+ B-cells within the lymphocytes was 28% (range, 4% to 53%). After 4 weekly rituximab infusion, the median CD19+ B-cell count decreased 0/μL (range, 0 to 6.04), and the median proportion was 0% (range, 0% to 0.27%).
2. Adverse events
Infusion-related complications were reported in nine of 32 patients (28.1%) (Table 3). There was no difference in the frequency of adverse events among disease subgroups. No infectious complications related to rituximab, including progressive multifocal leukoencephalopathy, were reported in our study group. All complications were below grade 3 CTCAE. Most symptoms related to adverse events were temporary or recovered after the infusion rate was slowed and symptomatic treatment with antihistamine and antipyretics as administered. No adverse events resulted in the withdrawal of rituximab.
Discussion
The results of this single center, retrospective study support the efficacy and safety of rituximab in pediatric autoimmune neuroinflammatory disorders.
Many previous studies have evaluated the efficacy of rituximab in autoimmune diseases of the nervous system. Dale et al. [14] performed a multicenter retrospective study of 144 children and adolescents. They analyzed the increased proportion of patients with mRS scores ≤2 at initiation of rituximab treatment and at last follow-up, and reported an improved outcome in 73.9% of patients at last follow-up. In another study, Fu et al. [21] also evaluated the efficacy of rituximab in 38 children and reported a good response in 74% of patients. Similarly, efficacy results in our study of 35 patients showed an improved outcome in 77.1% of patients at last follow-up. Hence, the overall efficacy of rituximab seen in our study is consistent with those seen in previous studies.
Several pediatric studies have explored the efficacy of rituximab in anti-NMDAR encephalitis, OMAS, and NMOSD. Our study results are also consistent with those of these previous studies. For anti-NMDAR encephalitis, a good outcome (mRS score 0 to 2) was reported in 78% and 90% of patients treated with rituximab at last follow-up [8,22]. We also demonstrated a comparable favorable outcome in 10 of 11 patients (91%) at a median 2 years follow-up. In patients with OMAS, an improved outcome (mRS score 0 to 2) was identified in 93.3% of patients at last follow-up [14]. Our study showed comparable favorable outcomes in 100% of patients at 2 months after initiation and at last follow-up. These results reflect the high efficacy of rituximab in refractory OMAS [23]. In NMOSD, a favorable outcome (mRS score 0 to 2) was reported in 90% of patients at last follow-up and there was a decrease in the annual relapse rate from 2.0 to 0.16 [14,24]. Although our study sample size was small, our data also showed a good outcome in two of four patients (50%) and also a decrease in the annual relapse rate (from 2.5 to 0.5). Considering both our study and those reported previously, the use of rituximab therapy in anti-NMDA encephalitis, OMAS, and NMOSD should be considered as soon as possible, and active use of rituximab is expected to improve the outcomes.
Although there were limited previous data about the effect of rituximab in patients with CIDP, the results in our study showed a promising trend. In patients with refractory CIDP, a previous study reported that two of three patients (66.6%) showed improvements in motor scale and morbidity [12]. Our study demonstrated a good outcome in one of two patients (50%) at both 2 months and at last follow-up. However, although we confirmed the consistently favorable outcomes, only a few small studies have explored rituximab in CIDP. Hence, further studies are needed to support the evidence for the efficacy of rituximab.
Previous studies reported rituximab infusion-related acute complications in 5% to 53% of patients with pediatric nephrotic syndrome [5,25,26]. In adult NMOSD, a systematic review and a meta-analysis reported infusion-related adverse events in 45 of 438 patients (10.3%) [27]. Infusion-related acute reactions were reported in 18 of 144 patients (12.5%) with pediatric autoimmune neuroinflammatory disorders [14]. Compared with this previous study, adverse events were observed in 10 of 35 patients (28.5%) in our study. This difference may be related to the method of classification of adverse events. The two previous studies did not include hematologic complications (anemia, thrombocytopenia, and neutropenia). If we exclude such hematologic complications, the adverse events rate decreases to 11.4% (four of 35 patients). The relatively smaller number of study patients might also magnify the results. Another small study of pediatric patients with central demyelinating disease showed infusion-related reactions in three of 11 patients (27.2%) [28]. Although we saw no difference between disease subgroups in the frequency of adverse reactions, it is necessary to consider this in the context of patients’ disease-related general condition. Nine patients with anti-NMDAR encephalitis and suspected autoimmune encephalitis developed fever with tachycardia before rituximab infusion, which continued during infusion. Because these events commenced before the infusion, we did not include them as infusion-related adverse events. Although there were some infusion reactions in our study, all adverse events were below grade 3 CTCAE and subsided after slowing of the infusion rate and administration of antipyretics. Overall, the data indicate that infusion-related adverse events of rituximab are well tolerated.
Previous studies have reported rituximab-related infectious adverse events including some serious infectious complications such as sepsis [14,26], Pneumocystis jiroveci pneumonia [21,29], hypogammaglobulinemia [14,30], and progressive multifocal leukoencephalopathy [29]. In one large pediatric cohort study, infectious complications occurred in 41 of 573 patients (7.3%) with autoimmune disease [29]. However, there were no infectious complications seen in our study. This result might be related to the small number of patients and relatively short follow-up period for evaluating infectious complications. It is also possible that patients might not have reported minor infectious events. Importantly, because previous studies have reported discontinuation of rituximab related to infectious adverse events, infectious complications should be monitored carefully during rituximab treatment and the acquisition of long-term safety data for rituximab in neuroinflammatory disease is required.
In our single center study, all patients received the same rituximab therapy protocol. Thus, our study data could provide powerful evidence for evaluating the efficacy and safety of rituximab. Our results have value in terms of the evidence supporting the efficacy and safety of rituximab treatment in pediatric autoimmune neuroinflammatory disorders. The accumulated data suggest that rituximab can be administered actively and we expect that rituximab will be of benefit in a range of neuroinflammatory diseases [31].
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.26815/acn.2019.00290.
The definition of each autoimmune neuroinflammatory disease
Protocol of first-line immunotherapy
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: YJK, YKS, and BCL. Data curation: WJK and SYK. Formal analysis: WJK and SYK. Funding acquisition: JHC and KJK. Methodology: YJK, YKS, and BCL. Project administration: HK, HH, and JEC. Visualization: YJK. Writing-original draft: YJK. Writing-review & editing: JHC, KJK, and BCL.
Acknowledgements
This work was presented at the Korean Pediatric Society Congress, 2019.