 |
 |
- Search
| Ann Child Neurol > Volume 32(2); 2024 > Article |
|
Abstract
Purpose
The clinical profile of seizures among children exhibits ethnic and geographical variations. The objective of this study was to examine the clinical, etiological, and demographic profiles of childhood seizures.
Methods
This was a hospital-based, cross-sectional study. Data were collected on the socio-demographic profile, details of the clinical presentation of seizure episodes, past history of meningitis, and neuroimaging (i.e., computed tomography [CT] scans), as well as the history of risk factors. Numbers, percentages, the chi-square test, and the Fisher exact test statistic were calculated. A P value of <0.05 was considered significant.
Results
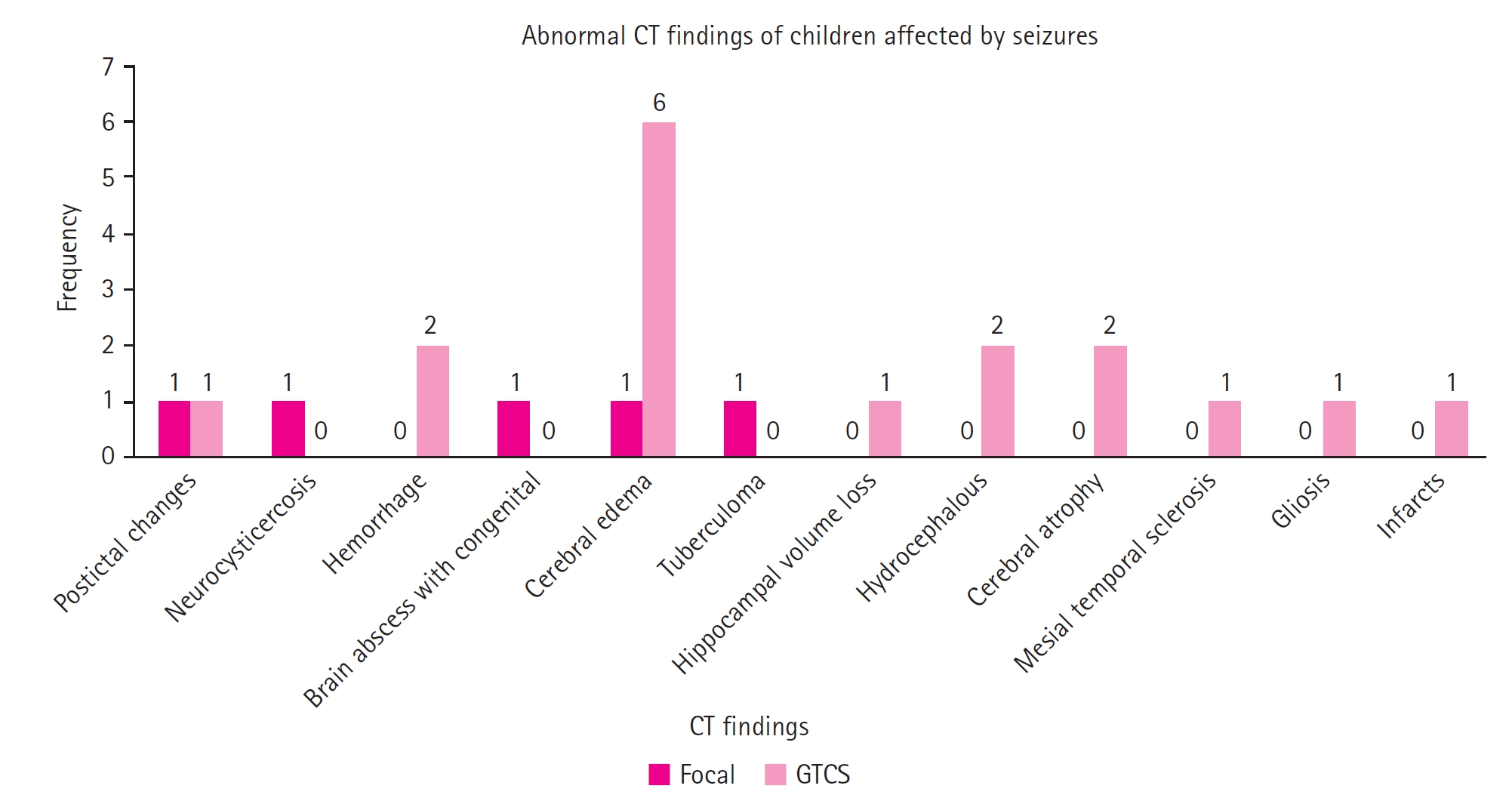
This study included 102 patients, of whom 82 experienced generalized tonic-clonic seizures (GTCS) and the remaining 20 had focal seizures. The most common age at presentation was between 1 and 4 years (55.9%). Approximately 70.0% of the children experienced postictal confusion and drowsiness, 38.2% had fever or sleep deprivation, and 25.5% suffered from headaches or vomiting. Postictal confusion and drowsiness were significantly more prevalent in children with GTCS (76.8%) compared to those with focal seizures (45.0%). Cerebral edema was the most common abnormality detected on CT scans in children with GTCS (n=6).
Conclusion
Younger age, neonatal brain insult, and family history were found to be associated with a higher risk of seizure episodes. Postictal confusion and drowsiness were identified as the most common clinical features. Postictal confusion and drowsiness were significantly more prevalent in GTCS compared to focal seizures. Cerebral edema was the most common abnormality observed in GTCS on CT neuroimaging.
Seizures are a significant reason for hospital admissions in young children in developing countries [1]. By the age of 16, 4% to 10% of children will have experienced seizures, and approximately one-fifth of those with unprovoked seizures may go on to develop epilepsy [2]. The incidence of epilepsy in children varies from 41 to 187 per 100,000. In developed countries, the prevalence of epilepsy in children is between 3.2 and 5.5 per 1,000, while in underdeveloped countries, it ranges from 3.6 to 4.4 per 1,000 [3].
Diagnosing, classifying, and managing seizures in children is a formidable challenge. This challenge is further compounded in hospitals with limited resources, where it becomes a more pressing concern. Therefore, the present study was designed to explore and investigate seizures in children. The study aimed to examine the various types of seizures and their associated correlates as reported in the existing literature. Seizures are associated with significant morbidity and mortality. The etiologies of seizures, which are crucial for clinical evaluation, treatment, and prognostic considerations, are based on recognized underlying causes. Seizures can range from benign and self-limited, requiring minimal treatment, to being the initial sign of a serious medical illness. Geographical variations often determine the common etiologies of seizures, underscoring the importance of conducting a clinical, etiological, and demographic analysis of seizures in children.
This study was conducted to investigate the clinical, etiological, and electrophysiological profiles of childhood seizures at a tertiary care hospital in the Gwalior District of Central India.
This cross-sectional observational study was conducted at Kamla Raja Hospital, Gajra Raja Medical College, Gwalior (Madhya Pradesh), and included children admitted over a period of 1 and a half years with seizures. The sample size was calculated based on the prevalence of pediatric seizures, which is 0.6%, with a 5% level of significance and 1.5% absolute precision. Using the formula for cross-sectional studies, the calculated sample size was 102. The inclusion criteria for patients were as follows: all children aged 1 to 14 years presenting with seizures, whose parents provided informed written consent. Exclusion criteria included children with severe head injuries requiring surgical intervention, intellectual disabilities, and those younger than 1 year or older than 14 years.
Study was started after receiving prior approval from the institutional ethical committee (L No: 102/IEC-GRMC/2020). Written informed consent was obtained from all patients.
All eligible children underwent examination, and detailed information was collected, including the duration of seizures, presence of postictal confusion, headache/vomiting, and trigger features such as sleep deprivation, fasting, exposure to flashing lights, delayed cry after birth, delayed developmental milestones, family history of seizures, neonatal seizures, previous history of meningitis, developmental regression, and history of head trauma. The International League Against Epilepsy (2017) classification system was employed to categorize the two types of seizures. Severe acute malnutrition is characterized by a very low weight-for-height/length (Z-score below -3 standard deviation [SD] from the median World Health Organization child growth standards), a mid-upper arm circumference of less than 115 mm, or the presence of nutritional edema. A computed tomography (CT) scan was performed on all eligible children, and the findings were documented.
Data were collected and entered into MS Excel sheets (Microsoft, Redmond, WA, USA) and graphically represented using figures. Descriptive statistics were presented as numbers and percentages, as well as means and SDs. To assess the association with categorical variables, the chi-square test and Fisher's exact test were applied. The analysis was conducted using SPSS version 26 (IBM Corp., Armonk, NY, USA). A P<0.05 was considered significant.
In the current study, 102 participants were enrolled. Among these subjects, 63.7% were male and 36.3% were female. A majority (55.9%) were aged between 1 and 4 years; 26.5% of the children were in the 5- to 9-year age group, and 17.6% were between 10 and 14 years old. Forty-nine children came from rural backgrounds, while 53 resided in urban areas. A family history of seizures was noted in 9.8% of the children. Regarding the distribution of seizure types, 80.4% of the children experienced generalized tonic-clonic seizures (GTCS), while 19.6% had focal seizures. Fifty-four children (52.9%) had seizures lasting less than 5 minutes. Seizure occurrences were reported to be exclusively during the daytime in 29 children (28.4%) and solely at nighttime in 23 children (22.5%) (Table 1).
Furthermore, 70.6% of children experienced postictal confusion and drowsiness, while 25.5% reported headaches or vomiting, and 38.2% had a history of fever or sleep deprivation. A history of taking antiepileptic therapy was noted in 22.5% of the children, with 11.8% regularly receiving the medication. Additionally, 8.8% had meningitis, 2.9% had experienced head trauma, 3.9% had a delayed cry, 13.7% had delayed milestones, 9.8% had a history of neonatal seizures, 5.9% had maternal comorbidity, and 7.8% exhibited pallor. The study found that 7.8% of the children were severely acutely malnourished and had microcephaly, as indicated by their head circumference. Conversely, 88.2% of the children were normally nourished, and 91.2% had a normal head circumference (Table 2).
Table 3 indicates that factors such as sex, age, area of residence, family history, duration of seizures, and time of seizures have no significant association with the type of seizure experienced.
Postictal confusion and drowsiness were significantly more common following GTCS, with a prevalence of 76.8%, compared to 45.0% after focal seizures. Other clinical features, including headache/vomiting, triggering factors, history of epileptic therapy, regular drug intake, meningitis, head trauma, delayed crying, delayed milestones, neonatal seizures, regression of milestones, and pallor, were not found to be associated with the type of seizure (Table 4).
Cerebral edema was observed in seven children (six with GTCS and one with focal seizures), followed by postictal changes in two children (one with focal seizures and one with GTCS). Hemorrhage was noted in two children with GTCS, hydrocephalus in two children with GTCS, and cerebral atrophy in two children with GTCS (Fig. 1).
This study demonstrated that seizures are most prevalent in children aged 1 to 4 years (55.9%), followed by those aged 5 to 9 years (26.5%), and 10 to 14 years (17.6%). Pradeep and Saritha [4] reported that 49.5% of patients who experienced seizures were in the 1- to 5-year age group, with the 5- to 10-year age group accounting for 20.5% of total cases, including 30% of cases occurring between 5 and 6 years of age. Another study on afebrile seizures revealed that 63% of children had their first seizure before the age of 5 [5]. The higher incidence of seizures in the younger age group (1 to 4 years) may be attributed to perinatal brain insults. Additionally, the brains of younger children may have more excitatory receptors and neurotransmitters compared to those of older children, which could be another contributing factor.
Out of 102 children, 65 were male (63.7%) and 37 were female (36.3%), with a male-to-female ratio of 1.8:1. A similar ratio was observed in a study conducted by Pradeep and Saritha [4]. Among the 200 children included in their study, 120 were male (60%) and 80 were female (40%). Poudel et al. [5] demonstrated that seizures were more common in males, with a male-to-female ratio of 3:2. Similarly, the study by Daoud et al. [6] found that seizures were more prevalent in males than females, with a ratio of 1.6:1.
Seizures were observed more frequently among children residing in urban areas (52%) compared to those in rural areas (48%). Adal et al. [7] conducted a study that revealed seizures were more common in children living in urban areas than in rural ones. Asadi-Pooya and Hojabri [8] also noted that a higher proportion of people with epilepsy resided in urban (55.6%) rather than rural areas (44.4%). These findings align with our study. Our study area was located in an urban setting, which may explain why a greater number of participants were from urban areas. Additionally, awareness and accessibility to the center may be higher among urban participants.
Regarding malnutrition and epilepsy, Crepin et al. [9] reported that 86.2% of subjects were malnourished, a finding that differs from our study, which identified 7.84% of subjects with severe acute malnutrition and 3.92% as underweight. A study [5] focusing on afebrile seizures found that out of 308 children, 31 had microcephaly, accounting for 10.06%. This is comparable to the findings of Chanpura and Mori [10], who reported a 6% incidence of microcephaly. These figures are similar to those of our study, which reported a microcephaly rate of 7.8%.
Record et al. [11] reported that 7.6% of the subjects in their study experienced neonatal seizures, 1.7% had central nervous system infections, and 2.4% suffered from trauma. Das et al. [12] revealed that 3.19% of the children in their study had tubercular meningitis, while Jagadeesan and Malai Arasu [13] found an 8% incidence of Central Nervous System (CNS) infections. In the current study, neonatal seizures were present in 9.8% of the children, and meningitis was found in 8.8%, which aligns with the findings of these studies. Chanpura and Mori [10] reported a 2% incidence of meningitis among children. The observed causal relationship may be due to seizures occurring as a sequela of meningitis. Poudel et al. [5] and Albaradie et al. [14] demonstrated in their studies that 29.16% and 56% of children, respectively, had delayed developmental milestones. In contrast, the present study found that 13.72% of children exhibited delayed developmental milestones.
Poudel et al. [5] observed that seizures were generalized in 79.2% of cases, partial in 14.0%, and unclassified in 6.8%. Sivanandam [15] conducted a study in Tamil Nadu and found that 65% of patients had generalized seizures, while 15% had focal seizures, indicating that generalized seizures were more prevalent compared to focal seizures, a finding similar to our study. Although focal seizures are more common in children than generalized seizures, this study excluded children with intellectual disabilities, which may account for the lower incidence of focal seizures reported.
Senbil et al. [16] reported that, of 102 children studied, only 45 underwent neuroimaging. The most common abnormal findings were cerebral edema and cerebral abscess, each accounting for 4.90% of cases. Similarly, in our study, cerebral edema was the most frequently observed abnormality. Jain and Mangal [17] investigated CT scan results in children with focal seizures. Their study included 172 children, of whom 65 had normal CT scans. Among the 107 children with abnormal findings, the most common was a single ring-enhancing lesion, present in 50.46% of cases. Other findings included multiple ring-enhancing lesions in 5.6%, cortical infarcts in 11.21%, dilated ventricles in 9.34%, encephalomalacia in 5.60%, calcifications in 3.73%, brain tumors in 1.86%, gliosis in 2.80%, and porencephalic cysts and subdural effusions, each constituting 1.86%. Chanpura and Mori [10] conducted research on the clinical and etiological profile of pediatric seizures. They concluded that cerebral atrophy was present in 6% of the cases. In contrast, our CT findings indicated that cerebral atrophy accounted for 8.3% of the abnormal CT findings.
The data reported in this study can be valuable in guiding clinical management, particularly in the selection of antiepileptic drug therapy and investigative procedures, especially in settings with limited resources. The ultimate goal is to improve patient outcomes.
Not all patients could undergo neuroimaging, such as magnetic resonance imaging, due to various constraints. Additionally, bedside electroencephalography (EEG) facilities were not available, so EEG was performed only after patients had clinically stabilized.
This study provides insights into the socio-demographic profile of children with seizure disorders within the study population. Factors such as younger age, neonatal brain insult, and a family history of seizures were associated with an increased risk of seizure episodes. Postictal confusion and drowsiness were identified as the most common clinical features. Notably, postictal confusion and drowsiness were significantly more prevalent in patients with GTCS than in those with focal seizures. Cerebral edema was the most frequently observed abnormality in CT neuroimaging for GTCS. These findings could aid practitioners in the early diagnosis of seizures in children.
Notes
Author contribution
Conceptualization: MV, GD, AG (Arvind Gupta), and AG. Data curation: MV, GD, and AG (Arvind Gupta). Formal analysis: DS. Methodology: MV, AG (Arvind Gupta), and DS. Project administration: AG (Arvind Gupta). Visualization: GD, AG (Arvind Gupta), and AG (Ajay Gaur). Writing-original draft: AG (Ajay Gaur). Writing-review & editing: GD, AG (Ajay Gaur), and DS.
Acknowledgments
The authors acknowledge the parents of all the children who participated in this study.
Fig.┬Ā1.
Neuroimaging (computed tomography [CT] scan) finding distribution of study participants. GTCS, generalized tonic-clonic seizure.
Table┬Ā1.
Baseline characteristics of the participants (n=102)
Table┬Ā2.
Clinical profile of children with seizures (n=102)
Table┬Ā3.
Association of the type of seizure with participantsŌĆÖ demographic characteristics
Table┬Ā4.
Association of the type of seizure with patientsŌĆÖ clinical and etiological profile
References
2. McAbee GN, Wark JE. A practical approach to uncomplicated seizures in children. Am Fam Physician 2000;62:1109-16.

3. Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord 2015;17:117-23.


4. Pradeep GC, Saritha HM. Study of clinical profile of children admitted with new onset seizures in a tertiary care urban hospital in South Bangalore. IP J Pediatr Nurs Sci 2021;4:21-5.

5. Poudel P, Parakh P, Mehta K. Clinical profile, aetiology and outcome of afebrile seizures in children. JNMA J Nepal Med Assoc 2013;52:260-6.



6. Daoud AS, Batieha A, Bashtawi M, El-Shanti H. Risk factors for childhood epilepsy: a case-control study from Irbid, Jordan. Seizure 2003;12:171-4.


7. Adal HD, Alemu K, Muche EA. Seizure control status and associated factors among pediatric epileptic patients at a neurologic outpatient clinic in Ethiopia. PLoS One 2021;16:e0259079.



8. Asadi-Pooya AA, Hojabri K. Risk factors for childhood epilepsy: a case-control study. Epilepsy Behav 2005;6:203-6.


9. Crepin S, Houinato D, Nawana B, Avode GD, Preux PM, Desport JC. Link between epilepsy and malnutrition in a rural area of Benin. Epilepsia 2007;48:1926-33.


10. Chanpura VR, Mori HT. A study of clinical, etiological and neurodevelopmental profile of epilepsy in children aged 0-5 years. Int J Contemp Pediatr 2021;8:1189.


11. Record EJ, Bumbut A, Shih S, Merwin S, Kroner B, Gaillard WD. Risk factors, etiologies, and comorbidities in urban pediatric epilepsy. Epilepsy Behav 2021;115:107716.


12. Das K, Das SK, Pradhan S, Sahoo PI, Mohakud NK, Swain A, et al. Clinical feature and outcome of childhood status epilepticus in a teaching hospital, Odisha, India. Cureus 2020;12:e10927.



13. Jagadeesan P, Malai Arasu R. Clinical profile of children with epilepsy: a cross-sectional study. Int J Contemp Pediatr 2020;7:2168-71.


14. Albaradie R, Habibullah H, Mir A, Alshammari AK, Alajmi MS, Alsubaie FA, et al. The prevalence of seizures in children with developmental delay. Neurosciences (Riyadh) 2021;26:186-91.



15. Sivanandam S. Childhood seizures: importance of electroencephalogram and neuroimaging [dissertation]. Coimbatore, IN: Coimbatore Medical College; 2009.
16. Senbil N, Sonel B, Aydin OF, Gurer YK. Epileptic and non-epileptic cerebral palsy: EEG and cranial imaging findings. Brain Dev 2002;24:166-9.


17. Jain N, Mangal V. Role of EEG and CT scan in partial seizures in children. Int J Med Med Sci 2011;3:161-3.







