 |
 |
- Search
| Ann Child Neurol > Volume 32(1); 2024 > Article |
|
Abstract
Purpose
This study was conducted to evaluate the correlation between persistent serum lactate elevation and brain magnetic resonance imaging (MRI) in children with status epilepticus (SE).
Methods
In this prospective analytical study, serum lactate levels were measured 24 hours after episodes of SE, and brain MRI was performed within 7 to 14 days after SE termination. MRI abnormalities were classified as acute encephalopathy (AE) grade I to III. The Kruskal-Wallis test was utilized for statistical analysis.
Results
The study included 42 participants with SE, of whom 85.70% were boys, with a mean age of 4.94 years. Viral encephalitis was the most common diagnosis, accounting for 47.60% of cases. Elevated serum lactate levels were detected in 71.40% of patients, and approximately 47.60% exhibited abnormal MRI findings consistent with AE grade I. The median serum lactate levels for AE grades I, II, and III were 1.50, 3.10, and 0.78 mmol/L, respectively. Two patients died, and 66.70% experienced neurologic sequelae. A significant correlation (P=0.021) was observed between persistent serum lactate elevation and abnormal brain MRI findings.
Status epilepticus (SE) is a life-threatening neurological condition with high mortality and morbidity in children. It manifests as either recurrent seizures without a return to normal consciousness or prolonged seizures lasting longer than 30 minutes [1]. In the management of SE, assessing the prognosis is crucial to avoid both overtreatment and long-term complications, as SE can arise from a variety of causes, including encephalitis [2-7]. Appropriate diagnostic and therapeutic interventions are anticipated to lower mortality rates and influence the prognosis [2-7]. The annual prevalence of SE in the pediatric population is estimated to be between 18 and 23 cases per 100,000 children, predominantly affecting neonates through children up to 5 years old, with a mortality rate ranging from 2% to 7% [3]. The overall incidence of convulsive SE among children is estimated at between 10 and 38 cases per 100,000 children each year [8,9].
Brain magnetic resonance imaging (MRI) is employed in the evaluation of SE to assess structural damage, ascertain the cause of seizures, and predict patient prognosis [10,11]. Reported physiological changes include cerebral edema, hyperperfusion, and changes to the blood-brain barrier. An imaging study revealed that brain abnormalities were detected in 20% of cases using computed tomography scans, whereas MRI of the brain revealed abnormalities in 58% of individuals with SE [1].
Numerous studies have examined the relationship between serum biomarkers and neurological disorders [12-14]. Serum levels of one biomarker, lactate, typically increase within the first few hours following a seizure. Once the seizure has ceased, lactate production declines, and lactate is rapidly cleared from the system. The amount of blood lactate reflects the extent of brain damage and is associated with poor prognosis in children [11]. Nass et al. [15] have reported that lactate is an effective neurological biomarker for generalized seizures, with elevated levels detected in nearly 90% of participants within 30 minutes after seizure cessation. Matz et al. [16] found that serum lactate levels collected within 2 hours after generalized tonic-clonic seizures were significantly elevated. Similarly, Calabrese et al. [17] observed that both cerebrospinal fluid and plasma lactate levels, collected within 12 hours after the end of a seizure, were significantly raised. These findings suggest that SE causes a notable increase in lactate levels, which may serve as an indicator of morbidity and mortality [12]. Several studies have noted elevated blood lactate levels and imaging abnormalities in children with hypoxic ischemic encephalopathy (HIE) [18]. However, no research has been conducted to explore the persistence of elevated serum lactate levels, obtained within 24 hours following SE, and their reflection in brain imaging patterns in children. The aim of this study is to investigate the correlation between persistently elevated serum lactate levels and abnormal brain MRI findings in children with SE.
A prospective study was conducted from June to November 2019, encompassing all new pediatric patients with SE in the emergency room of Dr. Soetomo General Academic Hospital.
The study included children with SE, ranging in age from 1 month to 12 years, who presented during the research period and for whom informed consent forms were completed. Patients were excluded if they had a history of seizures or traumatic brain injury within the previous 3 weeks, or if they had congenital anomalies of the central nervous system. The diagnosis of SE was confirmed according to the standard international classification, which includes patients who have experienced continuous seizures or multiple seizures without regaining consciousness for 30 minutes or longer [1-3].
Each participant underwent blood testing to measure lactate levels as well as a head MRI examination. Blood samples for the determination of persistent serum lactate were taken 24 hours following the onset of the seizure; these samples were drawn from arterial vessels and processed within 15 to 30 minutes. Subsequently, MRI of the head was performed 7 to 14 days after the seizure, as per a predetermined schedule, and included diffusion tensor imaging (DTI) sequences.
DTI is a modern imaging modality that can detect changes in the neuronal microstructure and other abnormalities not revealed by conventional MRI. Brain MRI examinations were conducted using magnetic resonance spectroscopy and perfusion with an MR360 Optima 1.5T system (GE HealthCare, Chicago, IL, USA). The MRI findings were reviewed by two neuroradiologists. MRI results for acute encephalopathy (AE) were classified into three categories based on the location of the observed abnormalities. Grade I was characterized by disorder confined to the white matter tracts, grade II encompassed both cortical and subcortical lesions, and grade III involved extensive lesions affecting the majority of the white matter [19-21].
Outcome evaluation was conducted at the time of patient discharge. Comprehensive data were documented, encompassing age, sex, nutritional status, history of comorbidities, hemoglobin level, leukocyte count, C-reactive protein level, serum lactate level, interpretation of head MRI, and duration of hospital stay. The outcomes were classified into three categories: ŌĆ£goodŌĆØ indicated no neurological sequelae; ŌĆ£mild to severe disabilityŌĆØ denoted the presence of mild to severe neurological sequelae; and ŌĆ£deathŌĆØ referred to patient mortality.
Ethical approval was obtained from the Ethical and Medico-legal Committee at Dr. Soetomo General Hospital Surabaya, under the ethics number 246/Panke.KKE/IV/2017.
All analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). We examined the association between serum lactate levels and brain MRI abnormalities in patients with SE by employing the Kruskal-Wallis test and the Spearman correlation test. A two-sided PŌēż0.05 was considered to indicate statistical significance.
A total of 44 participants presented with SE. All these patients were admitted through the emergency room before being transferred to the intensive care unit. Two patients died before a head MRI could be performed. The remaining 42 children, who fulfilled the inclusion criteria, underwent both serum lactate testing and brain imaging. The baseline clinical characteristics of the patients are detailed in Table 1.
The study participants ranged in age from 1 month to 12 years, with a mean age of 4.94 years. The majority were male, accounting for 85.70%. Regarding nutritional status, 30 participants (71.40%) were assessed as normal. Viral encephalitis was the most common cause of SE, accounting for 20 cases (47.60%), while epilepsy was identified as the etiology in 10 cases (23.80%). Of the 42 children with SE, 38 (90.40%) received treatment for more than 7 days in the hospital. Upon discharge, 12 cases (28.60%) had a favorable outcome, whereas 28 cases (66.70%) experienced outcomes ranging from mild to severe disability, and two cases (4.80%) were fatal.
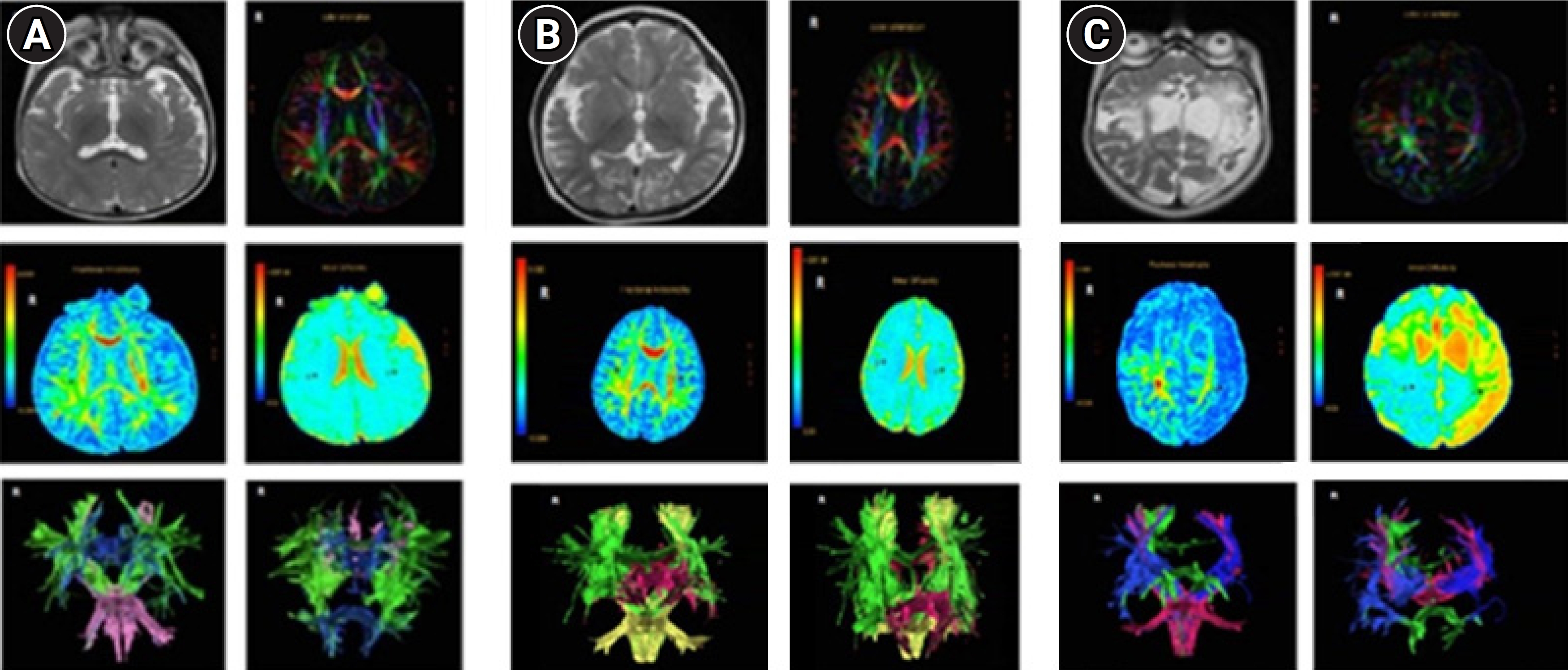
The data revealed that only two (4.80%) of the children with SE exhibited normal brain MRI results. The rest of the cohort presented with abnormalities on MRI, categorized as AE grade I (47.60%), grade II (28.60%), or grade III (14.30%). The macroscopic appearances of the head MRI samples are depicted in Fig. 1. Of the patients with SE, 71.40% demonstrated serum lactate levels above the normal range of 0.3 to 1.3 mmol/L.
A comparative analysis was conducted to examine the relationship between serum lactate level and the degree of encephalopathy. The extent of encephalopathy was assessed using MRI images. As shown in Table 2, the persistent serum lactate level was shown to increase with escalating AE grade. In patients with normal head MRI images, the median serum lactate level was 1.30 mmol/L. For those with AE grade I, the median serum lactate level rose to 1.50 mmol/L. Patients with AE grade II had a median serum lactate level of 3.10 mmol/L, and those with AE grade III had a median level of 3.50 mmol/L. The analysis indicated that serum lactate levels varied significantly with the grade of AE as determined by brain MRI (P=0.021). However, no significant relationship was observed between serum lactate levels and outcomes in children with SE, as presented in Table 3 (P=0.187).
We evaluated the associations of serum lactate levels, the degree of AE, and patient outcomes. The results of the Spearman analysis indicated a significant correlation between serum lactate level and the degree of AE (P=0.001, rs=0.674). A significant correlation was also observed between AE grade and patient outcome (P=0.004, rs=0.594).
Several studies have been published regarding the importance of imaging in SE. In brain imaging research, individuals with SE typically exhibit initial cerebral edema, followed by a progressive reduction in brain volume. The inner grey matter has been identified as the most vulnerable to damage from prolonged seizures [1,22,23]. MRI scans of the head using DTI sequences can reveal structural and functional damage in various brain regions associated with decreased consciousness due to seizures. Areas commonly impacted in both function and structure include the thalamus, brainstem, and consciousness pathways. In one study, the apparent diffusion coefficient values were significantly higher (P<0.05) in the bilateral dorsal thalamus and postero-superior midbrain of patients experiencing seizures. This suggests a disruption in the thalamus and upper brainstem, which are critical regions for indicating impaired consciousness in seizure patients [20,24-27]. DTI is a modern imaging modality capable of characterizing the random movement of water molecules, known as Brownian motion. It can be used to detect neuronal microstructural changes and abnormalities that conventional MRI may not reveal. Additionally, DTI offers the benefit of illustrating the microstructure of the central nervous system during brain development and maturation [20].
In this study, we employed head MRI examinations with contrast-enhanced DTI sequences to visualize neuronal damage resulting from SE seizures. Abnormalities on head MRI were observed in nearly all children with SE. The most common neuroimaging pattern identified was AE grade I. Gunawan et al. [19] reported findings consistent with ours, noting similar MRI abnormalities in children with SE. The distribution of MRI-detected encephalopathy in our study was as follows: grade I in 41.7% of cases, grade II in 33.3%, and grade III in 25% [19].
Blood lactate levels rise markedly within the first 60 minutes following brain damage. Some believe that this elevation is temporary, exhibited while the seizure is ongoing; afterwards, lactate production will decrease, and lactate will dissipate rapidly [14]. Correspondingly, serum lactate concentration may diminish over time. In cases of mild brain injury, serum lactate levels remain low, whereas in instances of severe injury, they decline over time [18]. Our study identified a sustained increase in serum lactate levels (measured over 24 hours following SE) exceeding 1.3 mmol/L. This increase is attributed to enhanced anaerobic glucose metabolism during hypoxic events associated with tonic-clonic seizures [15,16]. When the blood-brain barrier is compromised due to inflammationŌĆöstemming from infection, seizure-induced hypoxic lesions, or traumatic brain injuryŌĆölactate levels in the blood will rise. This response renders lactate a meaningful clinical biomarker for detecting brain cell damage observed during seizures [27].
Research exploring the relationship between persistent serum lactate levels and neuroimaging is still scarce. This study seeks to ascertain whether elevated lactate levels over an extended period in the blood of children with SE are correlated with brain imaging findings indicative of functional brain damage. The results indicate that serum lactate levels increase proportionally to the degree of AE. Our study revealed an association between sustained serum lactate levels and the severity of AE as determined by brain MRI. Cerebral blood flow initially rises during the early phase of a seizure and subsequently falls in the later stages as blood pressure drops. However, the brainŌĆÖs metabolic processes continue to require glucose and oxygen. The accumulation of lactate and depletion of adenosine triphosphate are associated with hypermetabolic neuronal necrosis. The excitotoxicity mechanism, mediated by glutamate N-methyl-D-aspartate (NMDA) and non-NMDA receptors, activates ion channels, leading to increased calcium permeability. This process contributes to neuronal damage in SE [28]. Elevated lactate levels can serve as a sensitive biomarker and predictor of brain damage. A study of neonates revealed evidence of increased serum lactate levels 72 hours after HIE with hypothermia treatment. Abnormal brain MRI findings among those neonates exhibited a correlation with poor neurological outcomes [15].
A prior study indicated that lactate serves as a prognostic factor for the incidence of SE, with higher blood lactate levels associated with poor outcomes [28-30]. Concurrently, the extent of encephalopathy as determined by head MRI findings in children with SE was found to significantly correlate with outcomes. The specific changes observed on head MRI reflect distinct patterns of brain injury (predominantly affecting the basal ganglia in cases of ŌĆ£acute-total,ŌĆØ and primarily involving watershed areas in ŌĆ£prolonged-partialŌĆØ or ŌĆ£severe-globalŌĆØ damage), and these changes are strongly associated with neurodevelopmental abnormalities [31].
One limitation of this study is that persistent elevations in serum lactate may be influenced by extracranial disorders. Consequently, additional research is warranted to compare blood lactate levels with those in cerebrospinal fluid, which would allow for the determination of lactate concentrations unaffected by extracranial conditions or other underlying diseases. Furthermore, respiratory issues present in the study participants were not specifically investigated, despite the possibility that hypoxia could be linked to impaired oxygenation. Moreover, lactate levels were not assessed using magnetic resonance spectroscopy. Future studies should examine the correlation between serum lactate and brain lactate levels in SE.
In conclusion, the study revealed an association between persistently elevated serum lactate levels and brain MRI abnormalities. Furthermore, the findings demonstrated that AE grade I represented the most common pattern of brain damage in patients with SE. Moreover, a correlation was identified between abnormalities on brain MRI and patient outcomes in children with SE. These findings are expected to be valuable in guiding the management and predicting the prognosis of patients who experience SE.
Notes
Author contribution
Conceptualization: PIG, RN, and SMS. Data curation: PIG. Formal analysis: PIG, RN, and SMS. Methodology: PIG and RN. Project administration: PIG, RN, and SMS. Visualization: PIG and SMS. Writing-original draft: PIG, RN, and SMS. Writing-review & editing: PIG, RN, and SMS.
Acknowledgments
The authors would like to thank the pediatric residents of our institution for supporting this study.
Fig.┬Ā1.
Brain magnetic resonance imaging visualization (T2-weighted images, axial view) of a patient with status epilepticus. (A) Acute encephalopathy (AE) grade I, characterized by disorder confined to the white matter tract. (B) AE grade II, also depicting disorder limited to the white matter tract. (C) AE grade III, involving a lesion impacting most of the white matter.
Table┬Ā1.
Baseline patient characteristics
Table┬Ā2.
Relationship between persistent serum lactate elevation and AE grading on magnetic resonance imaging in children with status epilepticus
| AE grading | Persistent serum lactate level | ||||
|---|---|---|---|---|---|
| Number | Median | Min | Max | P value | |
| Normal | 2 | 1.30 | 1.30 | 1.30 | |
| AE grade I | 20 | 1.50 | 0.70 | 3.20 | 0.021a |
| AE grade II | 12 | 3.10 | 2.20 | 5.30 | |
| AE grade III | 8 | 3.50 | 2.70 | 4.20 | |
References
1. Smith DM, McGinnis EL, Walleigh DJ, Abend NS. Management of status epilepticus in children. J Clin Med 2016;5:47.



2. Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48-61.




3. Moghaddasi M, Joodat R, Ataei E. Evaluation of short-term mortality of status epilepticus and its risk factors. J Epilepsy Res 2015;5:13-6.



5. Kushawaha SK, Raj N, Sinha M, Kumar P, Ashawat MS. Nipah virus and its outbreaks in tropical areas. Res J Pharm Technol 2020;13:491-7.

6. Sen DJ, Patel JS, Garg CS, Shah DH, Patel KM, Bhavsar DS, et al. Mosquito sting: a host of parasites and virions. Res J Sci Technol 2011;3:119-26.
7. Pujar SS, Neville BG, Scott RC, Chin RF; North London Epilepsy Research Network. Death within 8 years after childhood convulsive status epilepticus: a population-based study. Brain 2011;134(Pt 10):2819-27.



8. Barzegar M, Shiva S, Rahbari-Banaeian G. Etiology and short-term outcome of children with convulsive status epilepticus admitted to Tabriz ChildrenŌĆÖs Hospital, Iran. J Anal Res Clin Med 2014;2:112-7.
9. Kumar M, Kumari R, Narain NP. Clinical profile of status epilepticus (SE) in children in a tertiary care hospital in Bihar. J Clin Diagn Res 2014;8:PC14-7.



10. Vestergaard MB, Lindberg U, Aachmann-Andersen NJ, Lisbjerg K, Christensen SJ, Law I, et al. Acute hypoxia increases the cerebral metabolic rate: a magnetic resonance imaging study. J Cereb Blood Flow Metab 2016;36:1046-58.




11. Chiang MC, Lien R, Chu SM, Yang PH, Lin JJ, Hsu JF, et al. Serum lactate, brain magnetic resonance imaging and outcome of neonatal hypoxic ischemic encephalopathy after therapeutic hypothermia. Pediatr Neonatol 2016;57:35-40.


12. Pratamastuti D, Indra Gunawan P, Saharso D. Serum neuron specific enolase is increased in pediatric acute encephalitis syndrome. Korean J Pediatr 2017;60:302-6.




13. Ibrahim DE, Alhashemi WK, Alhussaini SI. Study of docosahexaenoic acid and eicosapentanoic acid effects on some biochemical parameters in epileptic patients. Res J Pharm Technol 2020;13:319-22.

14. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc 2013;88:1127-40.



15. Nass RD, Zur B, Elger CE, Holdenrieder S, Surges R. Acute metabolic effects of tonic-clonic seizures. Epilepsia Open 2019;4:599-608.




16. Matz O, Zdebik C, Zechbauer S, Bundgens L, Litmathe J, Willmes K, et al. Lactate as a diagnostic marker in transient loss of consciousness. Seizure 2016;40:71-5.


17. Calabrese VP, Gruemer HD, James K, Hranowsky N, DeLorenzo RJ. Cerebrospinal fluid lactate levels and prognosis in status epilepticus. Epilepsia 1991;32:816-21.


18. Wu TW, Tamrazi B, Hsu KH, Ho E, Reitman AJ, Borzage M, et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front Neurol 2018;9:293.



19. Gunawan PI, Saharso D, Sari DP. Correlation of serum S100B levels with brain magnetic resonance imaging abnormalities in children with status epilepticus. Korean J Pediatr 2019;62:281-5.




20. Trivedi R, Rathore RK, Gupta RK. Review: clinical application of diffusion tensor imaging. Indian J Radiol Imaging 2008;18:45-52.


21. Meletti S, Monti G, Mirandola L, Vaudano AE, Giovannini G. Neuroimaging of status epilepticus. Epilepsia 2018;59 Suppl 2:113-9.



22. Tsuchida TN, Barkovich AJ, Bollen AW, Hart AP, Ferriero DM. Childhood status epilepticus and excitotoxic neuronal injury. Pediatr Neurol 2007;36:253-7.


23. Goothy SS, Goothy S. Assessment of anticonvulsant properties of caloric vestibular stimulation in pilocarpine-induced status epilepticus mice model. Res J Pharm Technol 2021;14:405-8.

24. Patel JB, Patel KM, Shah DH, Patel JS, Garg CS, Brahmbhatt KJ, et al. Functional magnetic resonance imaging: a new diversion in medical diagnosis. Res J Pharm Technol 2011;4:1167-76.
25. Burje S, Rungta S, Shukla A. Detection and classification of MRI brain images for head/brain injury using soft computing techniques. Res J Pharm Technol 2017;10:715-20.

26. Dayani MA, Daneshi A, Ahadi R, Shekarchi B, Fatehi D. Diagnostic value of magnetic resonance spectroscopy in morphometrical analysis of basal ganglia in patients with idiopathic generalized epilepsy. Res J Pharm Technol 2017;10:2693-6.

27. Xie F, Xing W, Wang X, Liao W, Shi W. Altered states of consciousness in epilepsy: a DTI study of the brain. Int J Neurosci 2017;127:667-72.


28. Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 1993;34 Suppl 1:S37-53.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,808 View
- 29 Download
- Related articles in Ann Child Neurol
-
Brain Magnetic Resonance Imaging in Children with CNS Manifestations.2005 May;13(1)
The Correlation between ADHD and Brain MRI Findings in Children with Epilepsy.2018 June;26(2)
Correlation between the Handedness and Clinical Findings in Children with Epilepsy.2001 May;9(1)






