 |
 |
- Search
| Ann Child Neurol > Volume 31(2); 2023 > Article |
|
Abstract
Purpose
This study aimed to determine the clinicoepidemiological profile and prognostic factors in children with neurotuberculosis.
Methods
An observational study was conducted at a tertiary care hospital on 50 children diagnosed with neurotuberculosis. The demographic profile, clinical details, and all investigations were recorded on a predetermined form and datasheet for analysis, and disability severity was graded using the modified Rankin Scale.
Results
Fifty patients were enrolled (male-to-female ratio, 1.08:1). Younger children (1 to 3 years) were more commonly affected. Most patients were malnourished, and only 58% had been immunized with the Bacillus Calmette-Guerin vaccine. Fever was the most common symptom (96%) followed by headache, altered consciousness, vomiting, seizures, and abnormal posture. On cerebrospinal fluid (CSF) analysis, 87% of patients showed pleocytosis with lymphocytic predominance. More than 80% of patients had CSF protein levels >100 mg/dL and CSF glucose levels <60 mg/dL. Common findings on neuroimaging were hydrocephalus (76%), basal meningeal enhancement (60%), basal exudates (52%), and parenchymal infarcts (32%). We noted a mortality rate of 40%, of whom 87.5% had modified British Medical Research Council (BMRC) stage 3 tubercular meningitis (TBM). All patients who survived stage 3 TBM had a severe disability, and over 50% of patients with stage 2 TBM had moderate to severe disabilities.
Globally, the annual incidence of tuberculosis in recent years is relatively stable, with an estimated number of 10 million (range, 9 to 11.1 million) people affected in 2018 [1]. The incidence is quite variable among different countries, ranging from <5 to >500 new cases per 100,000 population per year, with a global average of 130.1. Eight countries accounted for nearly two-thirds of all cases globally, with India (27%) and China (9%) leading the counts [1].
Globally, 11% of tuberculosis cases were recorded in children less than 15 years of age [1]. Almost one-fourth of children with tuberculosis have extrapulmonary tuberculosis, the most serious type of which is tubercular meningitis (TBM) [2]. Younger children are more commonly affected by TBM, with a peak between 23 and 49 months [3-5]. Although TBM constitutes a small number of overall tuberculosis cases, it causes a disproportionate degree of mortality and morbidity [6]. The mortality rate among children with TBM varies from 13% to 70%, and only 16% to 20% of children are reported to have survived without any sequelae [3,7-18]. The majority of TBM survivors experience some residual neurological, cognitive, behavioral, or developmental impairment [3,10-17,19-21].
Although multiple studies have been conducted on neurotuberculosis in children, only limited studies have been carried out in the last decade regarding the clinicoepidemiological profile and prognostic factors in pediatric neurotuberculosis, which includes TBM, intracranial tuberculoma, spinal intramedullary tuberculosis, and Pott’s spine. Therefore, we conducted this study with the aim of ascertaining the clinical profile and prognostic factors in children with neurotuberculosis.
This cross-sectional study was conducted at the Department of Pediatrics of a tertiary hospital located in New Delhi, India, and 50 children between 1 month and 12 years of age diagnosed with neurotuberculosis were included. Approval from the Institutional ethical committee of Maulana Azad Medical College, New Delhi, India was granted before proceeding with the study (F no. 17/IEC/MAMC/2018/Paeds/19). Written informed consent/assent (depending on the case) was obtained from the subjects. A case was diagnosed as definite or suspected TBM and included in the study using the criteria and scoring system proposed by an international expert panel in 2010 based on clinical, cerebrospinal fluid (CSF), neuroimaging, and other supportive laboratory parameters specified for children. Specifically, a definite case of TBM was defined when acid-fast bacilli (AFB) were demonstrated in CSF by staining, culture, polymerase chain reaction, or cartridge-based nucleic acid amplification test (CBNAAT) [22].
Patients’ demographic details, presenting complaints, history of contact with a tuberculosis case, treatment history, and compliance of contact to antitubercular therapy (ATT), as well as whether the contact had multidrug-resistant tuberculosis were recorded. Whether a family had poor or low socioeconomic status was defined using the modified Kuppaswamy scale. A physical examination was performed, with a special emphasis on fever, Bacillus Calmette-Guerin (BCG) vaccine scar, clubbing, jaundice, hepatosplenomegaly, chest, and central nervous system (CNS) examinations, British Medical Research Council (BMRC) staging, and fundus.
The investigations done in each patient were a complete blood count, chest X-ray, and gastric aspirate (GA) CBNAAT to look for evidence of pulmonary tuberculosis; contrast-enhanced computed tomography and magnetic resonance imaging to establish the diagnosis; microscopic, biochemical, and microbiological CSF analyses; CSF CBNAAT (wherever possible and indicated); a liver function test to detect deranged liver function at admission and ATT-induced hepatitis; a tuberculin test, and an human immunodeficiency virus (HIV) test. The patients were monitored during the hospital stay, and we recorded instances of mortality, the time from admission to mortality, the need for neurosurgical interventions (e.g., a ventricular tap, external ventricular drainage, and ventriculoperitoneal shunt), the need for ventilation, and the development of ATT-induced hepatitis. At discharge, every patient was scored on the modified Rankin Scale to grade the severity of disability.
Data were entered in a Microsoft Excel spreadsheet and analyzed using SPSS for Windows version 25.0 (IBM Corp., Armonk, NY, USA) and analyzed using appropriate statistical tests.
In total, 50 patients with neurotuberculosis were enrolled from May 2019 to March 2020 after receiving consent from their guardians. Twenty-six (52%) of the patients were boys, with a male-female ratio of 1.08:1. Children younger than 5 years of age comprised 48% of cases. The maximum number of cases was in the age group of 1 to 3 years, comprising 30% of all cases. Only four out of 50 cases (8%) were younger than 1 year of age. Thirty (60%) children belonged to families where the most educated person in the family had either no education or was educated until the primary or secondary level. Forty-four (88%) of the patients belonged to either lower or lower-middle-class families. Seventy-five percent of the children <5 years of age and 55% of those >5 years of age were malnourished. Almost half of the children below 5 years of age were stunted, half were wasted, and one-third had severe malnutrition. Out of the 50 patients, only 29 (58%) patients had received BCG vaccination. A BCG scar was noted in 29 (58%) patients. It was seen that 26 (52%) patients had contact with a tuberculosis case. The demographic details are shown in Table 1.
The median duration of illness was 17 days (range, 4 to 150). The most important symptom of neurotuberculosis was fever, which was seen in 48 out of 50 (96%) cases. Headache was present in 82% (28/34) of the patients who were able to describe it. The median duration of headache was 2 weeks. The other important symptoms were altered consciousness, vomiting, and seizures. Seizures were present in 66% of the patients. Most of the patients had generalized tonic-clonic seizures. Irritability and weight loss were present in 64% and 38%, respectively. About one-third (36%) of patients presented with a paucity of limb movement. Elevated intracranial tension (ICT) and altered sensorium were seen in 86% and 80% of cases, respectively. Other symptoms and signs are tabulated in Table 2. Out of 50 cases of neurotuberculosis, TBM was seen in 46 (92%) patients. The remaining four cases had tuberculoma only. Twenty of the 50 (40%) patients had concomitant tubercular pneumonia diagnosed by supportive findings on the clinical examination and chest X-ray.
Out of 47 cases where a lumbar puncture was performed, 41 (87%) patients showed CSF pleocytosis. Thirty-three (70%) showed total cell counts in the CSF between 10 and 400 cells/mm3. Nineteen (40.4%) patients had CSF lymphocytosis of over 90%, and 29 (62%) patients had CSF lymphocytosis of more than 50%, 13 of whom had lymphocytes only. Twelve patients had a neutrophilic predominance in CSF. CSF glucose levels less than 60 mg/dL were seen in 85% of cases. The CSF was acellular in six patients, although all of them had low glucose, elevated protein, or both in the CSF. Out of the 46 patients in whom CSF CBNAAT was performed, the results were positive in 14 (30.4%) patients, who were labeled as definite TBM cases. Rifampicin (RIF) resistance was seen in two out of these 14 patients. GA CBNAAT was positive in only seven out of 49 cases (14.3%) (Table 3).
The commonest finding on neuroimaging was hydrocephalus, which was seen in 38 (76%) patients. Communicating hydrocephalus was more frequently observed than other types, with a presence in two-thirds of those with hydrocephalus. Basal enhancement was seen in 30 (60%) patients. Basal exudates, periventricular ooze, and infarcts were seen in 52%, 36%, and 36% of patients, respectively (Table 3 and Fig. 1).
Patients were started on ATT, and the regimen used was 2 months of isoniazid, RIF, pyrazinamide, and ethambutol followed by 7 months of isoniazid and RIF with ethambutol, for a total of 9 months as per the national policies. The corticosteroids used were intravenous dexamethasone for the first week, then oral prednisolone for 4 weeks, followed by tapering. Antiepileptics were used when required. Elevated ICT was managed medically using mannitol, hypertonic saline, and acetazolamide, and 11 patients required surgical management when medical measures failed to control elevated ICT. Six patients (12%) underwent ventricular tap. Four patients required external ventricular drainage, and one patient underwent ventriculoperitoneal shunt insertion. Sixteen patients (32%) who were either terminally ill, making any neurosurgical intervention inappropriate, or experienced sudden neurological deterioration needed mechanical ventilation.
The outcomes of the patients were very poor. Twenty out of 50 (40%) patients with neurotuberculosis died. The patients who survived were evaluated for severity of disability by the modified Rankin Scale at discharge (Table 4). Both surviving patients with stage 3 disease had moderate to severe disabilities at discharge. Out of 23 patients in stage 2, 14 were discharged with a Rankin Scale of 1, indicating no significant disability, while four patients had severe disability. The only patient with stage 1 disease was discharged with infrequent episodes of mild headache only. The factors associated with mortality were analyzed (Table 5).
In our study, 50 patients with neurotuberculosis were enrolled, with a slight male predominance. A male predominance of TBM in children has also been observed in other studies [10,13,15,23]. The youngest child affected was 5 months old, the age range was 5 to 134 months, and the median age was 60 months. In other studies, the median age also varied from 30 to 60 months [9,11,12,14,17,24]. We found that 60% of children’s families had a poor education and belonged to the lower socioeconomic class. In a study done by Israni et al. [13], 85% of the patients belonged to lower socioeconomic status families. Other studies have also reported similar findings [12,17]. Around 75% of the children less than 5 years of age and 55% of those more than 5 years were found to be malnourished. Girls contributed to a higher proportion of malnourished patients than boys in both age groups. This gender distribution of malnutrition has not been mentioned in other studies on pediatric TBM, but it might indicate the persistence of gender discrimination within lower-socioeconomic status families.
The most common symptom of neurotuberculosis was fever (96%), followed by headache (82%), altered consciousness (80%), vomiting (72%), and seizures (66%). Two patients presented without fever, of whom one had only tuberculoma and the other had TBM with tuberculoma. Patients with only tuberculoma are less likely to have fever, as has been shown in other studies as well [25,26]. Altered consciousness varied from mild confusion in some patients to a comatose state in a few. Most of these patients developed altered consciousness after the onset of seizure. Seizures were also common; generalized tonic-clonic seizures were most frequently seen, but patients having tuberculomas or infarcts were more likely to have focal seizures. The unusual complaints were visual loss (16%), and abnormal body movements in the form of choreoathetosis movements (6%). All three patients with choreoathetosis movements had developed an abnormal posture in the form of either decerebrate or decorticate rigidity before the onset of such movements. Dyskinesia has been described as a complication of TBM, mostly in case series and reports in the literature [27,28]. In a study by Alarcon et al. [27], 30 patients had movement disorders out of 130 TBM cases studied over 7 years. Twenty patients had tremors, seven had chorea, and three had dystonia. Out of seven patients, five were less than 5 years old and one was less than 12 years of age. Chorea has been described as an uncommon complication in children with TBM. The pathophysiology causing movement disorder appears to be multifactorial, and the sites of the lesions were found to involve the deeper part of the brain, usually the thalamic and subthalamic areas. However, these small deeper lesions are not always detected on neuroimaging. In our patients who had chorea, no focal lesions were found on neuroimaging. It is postulated that brain edema and bacterial toxins may cause neurotransmitter dysfunction in the basal ganglia, causing dyskinesia [29].
The most commonly affected cranial nerve was the seventh cranial nerve (22%), followed by the third (14% cases) and sixth (10%). In other studies as well, cranial nerve deficits are present in 12% to 46% of patients [4,9-15,30-32]. However, it was classically taught that the sixth nerve is the most commonly affected nerve due to its course inside the cranium. Cranial nerve deficits are a manifestation of brain stem involvement where basal exudate extends and infiltrates, and could also occur secondarily to the mass effect caused by a tuberculoma or an abscess. Elevated ICT can also produce a false-positive cranial nerve defect. Further extension of exudates around cerebral blood vessels causes vasculitis and vasospasm, resulting in motor deficits. Hemiparesis was seen in 11 patients, of whom seven patients had hemiparesis on the right side. Paraparesis was seen in one patient. Abnormal posture in the form of decerebrate or decorticate rigidity was seen in 11 patients, which again indicates the stage of the disease in which patients presented to us.
Out of 47 patients who underwent CSF evaluations, 41 (87%) showed CSF pleocytosis, predominantly lymphocytic. Twelve patients had a neutrophilic predominance in CSF. Five out of these 12 (42%) patients died, which suggests that the patients with CSF neutrophilia in our study did not have a better prognosis. This was in contrast to some studies that have shown a better prognosis in these patients [9,24]. CSF protein levels over 100 mg/dL were seen in 80% (38/47) of patients. A protein level >100 mg/dL has been shown to have a sensitivity of 0.78 for TBM in children [33]. CSF glucose levels less than 60 mg/dL were seen in 85% of cases. This aligns with other studies, where more than 80% of patients had CSF glucose levels less than 60 mg/dL [9-11,13,14,16]. Furthermore, two out of six (33.3%) patients with acellular CSF had positive CSF CBNAAT, which suggests a similar likelihood of CBNAAT positivity in those with and without pleocytosis. GA CBNAAT was positive in only seven out of 49 cases (14.3%). There is a scarcity of studies reporting GA CBNAAT findings in pediatric TBM, although Bang et al. [14] reported GA AFB positivity in 7% of patients.
Xpert Mycobacterium tuberculosis (MTB)/RIF is an automated diagnostic test that can identify MTB DNA and resistance to RIF. For children with TBM, the specificity and sensitivity for the Xpert MTB/RIF test were 100% and 39%, respectively, in children [34]. An important advantage of Xpert MTB/RIF is that it can detect RIF resistance within 2 hours. Bhatia et al. [35] reported the sensitivity of GeneXpert and BACTEC Culture (BD, Franklin Lakes, NJ, USA) as 38.25% and 14.71%, respectively, in children suspected of TBM. Nucleic acid amplification tests (NAAT) in the CSF are another new diagnostic tool for the detection of TBM [34]. A meta-analysis of 14 studies that evaluated the accuracy of NAAT in TBM diagnosis reported a sensitivity of 56%, a specificity of 98%, a negative likelihood ratio of 44, and a positive likelihood ratio of 35.1, suggesting that NAAT may play a significant role in the confirmation of tuberculosis, but not in ruling out TBM [36]. The World Health Organization recommends the use of Xpert MTB/RIF as the initial diagnostic test for CSF testing in children suspected of TBM. The limitations of using Xpert for the diagnosis of TBM include false negatives, which could lead to missed or delayed diagnoses, leading to poor outcomes. In our study, CSF CBNAAT was positive in 30.4% of patients, which is similar to what has been reported in studies published in various parts of the world. RIF resistance was seen in two out of these 14 patients (14.2%). In studies from Africa, RIF monoresistance was found in 5% of pediatric TBM cases [37]. There is a paucity of literature on the exact prevalence of RIF resistance in children with CNS tuberculosis.
Studies have shown that hydrocephalus and meningeal enhancement are important signs of TBM, observed in 80% and 75% of children with TBM [38]. Neuroimaging findings such as hydrocephalus (communicating type), basal enhancement, and exudates were seen in the majority of the patients. The results of these parameters in this study are comparable with those done in the past.
All patients with elevated ICT were started on medical management. Patients who did not respond optimally to medical management or had worsened ICT required neurosurgical intervention or ventilation. In our study, 11 out of 38 (29%) patients required neurosurgical interventions, which is a lower rate than reported in other recent Indian studies, where neurosurgical interventions were required in 50% to 90% of patients with hydrocephalus [9,10]. This might have been due to a variable degree of elevated ICT at presentation, as most of the patients could be managed by medical management alone, while some other patients succumbed within hours of presentation before any neurosurgical intervention could be done.
The outcomes were very poor, as 20 out of 50 (40%) patients with neurotuberculosis died. The proportion of mortality in TBM patients was higher (20/46, 43.5%), as no patient with only tuberculoma died. These deaths might have been due to late presentations and BMRC stage 3 TBM contributing to a higher proportion (one-third) of overall cases. The patients who survived were evaluated for the severity of disability using the modified Rankin Scale at discharge.
The factors associated with mortality were analyzed. Younger age, lower socioeconomic status, altered sensorium, stages 2 and 3 at presentation, poor Glasgow Coma Scale (GCS), abnormal posture, hydrocephalus, and the presence of basal exudates were associated with a higher risk of mortality. The mean age of the patients who died was 43 months, which was significantly lower than the mean age of 78 months for those who survived (P=0.007). Using a cutoff of 3 years for analysis, the mortality rate in those younger than 3 years of age was significantly higher (P=0.007). Patients younger than 3 years of age were 5.1 times more likely to die. The higher risk of mortality at a younger age can be attributed to late presentations due to non-specific clinical features, as evidenced by the fact that all four patients below 1 year of age presented with stage 3 TBM and all died. Two-thirds of patients belonging to lower socioeconomic status families died, while the same proportion of patients from middle-class families survived (P=0.031). This might have been due to a lack of awareness, poverty, crowding, malnutrition, and lack of immunization being more prevalent in such families. Nineteen out of 20 patients who died had altered sensorium at presentation, and 19 out of 40 (47.5%) patients with altered sensorium died (P=0.03). The BMRC stage at admission was found to be a very strong predictor of mortality (P<0.001), as also seen in the majority of other studies [7-10,13,14,24]. In total, 14 out of 16 (87.5%) stage 3 patients died. The two surviving patients with BMRC stage 3 TBM had significant residual disabilities, while the only patient with BMRC stage 1 TBM was discharged with minimal symptoms. Twenty percent of BMRC stage 2 patients died, and 60% of those who survived were discharged with minimal symptoms.
GCS at admission was also found to be a strong predictor of mortality apart from the stage (P<0.001). Both the patients with a GCS of less than seven died and only two out of 14 (14%) patients with a GCS of less than 10 could survive. Of surviving patients, the minimum GCS was 9. Some other studies also have shown GCS as an important prognostic factor [13,15]. All four patients with decerebrate posture died.
Patients with hydrocephalus were much more likely to die than patients without hydrocephalus (P=0.007), as 50% of patients with hydrocephalus died versus 8% of those who did not have hydrocephalus. The presence of obstructive hydrocephalus imposed an even greater risk of mortality, as only one out of six patients with obstructive hydrocephalus survived. That patient was discharged with significant disability (modified Rankin Scale of 4) despite having BMRC stage 2 TBM at presentation. The other two radiological findings associated with increased mortality were the presence of basal exudates (P=0.038) and periventricular ooze (P<0.001). Fourteen of the 26 (54%) patients with basal exudates died. Only three out of 18 (17%) patients with periventricular ooze survived. Out of the 15 patients with periventricular ooze who died, 12 required mechanical ventilation to control ICT. None of the patients with tuberculoma (with or without TBM) died (P=0.001). This might indicate better immunity in these patients and thus a better prognosis. This is further supported by the fact that only one patient with tuberculoma had BMRC stage 3 TBM, and that patient also survived despite presenting after 3 months of illness.
Most other studies have shown similar findings, except for elevated ICT, focal deficits, and infarcts, which have been shown to predict mortality and morbidity [7-10,14-16,24]. Conflicting results have been reported regarding CSF profiles [9,10,13,14]. Though all five patients with ATT-induced hepatitis survived, this might have been because most pediatric patients have been shown to develop this condition at or after 1 week of therapy, and most of the deaths in our study occurred before that [39,40]. Thus, rather than being a prognostic factor, ATT-induced hepatitis may have developed only in patients who survived long enough to develop it.
Recent data regarding the clinical spectrum and outcomes of pediatric extrapulmonary tuberculosis, especially TBM, from tertiary centers in India are limited. The clinical features of pediatric TBM often resemble CNS infections due to viral, bacterial, and fungal infections. Children often present in the late stage of the disease, which is associated with higher morbidity and mortality. The clinical staging at presentation is a major factor determining mortality. Hence, a high degree of suspicion is needed for early diagnosis and management. Children are at high risk for neurocognitive and behavioral disabilities, especially if diagnosed late.
In children, clinical features remain inconclusive for diagnosing neurotuberculosis, especially TBM. Neuroimaging appears to be the most sensitive tool for both diagnosis and prognosis. Young age, lower socioeconomic status, BMRC stages 2 and 3, abnormal posture, hydrocephalus, and the presence of basal exudates were found to be associated with poor outcomes in this study. Hence, a high level of suspicion for neurotuberculosis in children must be maintained to prevent neurological morbidity, disability, and mortality.
This study has several strengths. First, this is one of the few studies that has been conducted on prognostic factors of pediatric TBM patients in India in the last decade. Second, some new prognostic factors, such as abnormal posture, periventricular ooze and tuberculoma, emerged from this study, although these findings require further confirmation. Third, this is one of the few studies to include GA CBNAAT in the pediatric TBM evaluation. Fourth,neuroimaging was found to be the most sensitive diagnostic modality in our study, thus highlighting the role of early neuroimaging in pediatric TBM.
Notes
Author contribution
Conceptualization: KR. Data curation: VS. Formal analysis: VS and DK. Methodology: VS and KR. Visualization: KR. Writing - original draft: VS and KR. Writing - review & editing: DK and GG.
Fig. 1.
Common neuroimaging features of tubercular meningitis (arrows). (A) Hydrocephalus with periventricular ooze. (B) Basal exudates. (C) Infarct. (D) Tuberculoma.
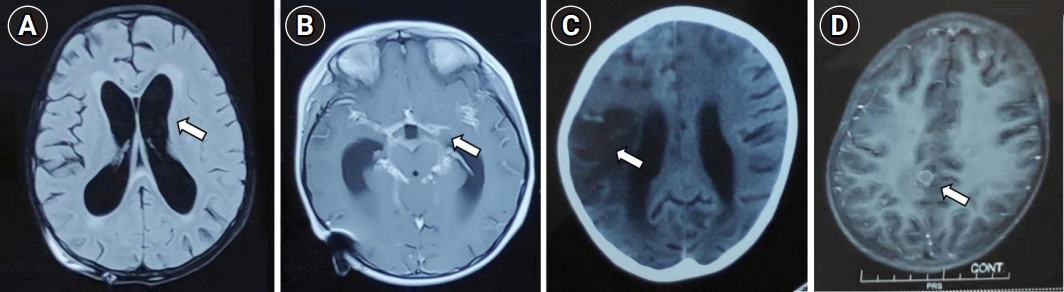
Table 1.
Patients’ demographic profile
Table 2.
Clinical signs and symptoms
Table 3.
Laboratory findings
Table 4.
Modified Rankin Scale at discharge of those who survived (n=30) in different stages
Table 5.
Factors associated with mortality (total mortality of 20 patients out of 50 included patients)
References
1. World Health Organization. Global tuberculosis report 2019 [Internet]. Geneva: WHO; 2019 [cited 2023 Jan 17]. Available from: https://www.who.int/publications/i/item/9789241565714
2. Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health 2014;2:e453-9.


3. van Well GT, Paes BF, Terwee CB, Springer P, Roord JJ, Donald PR, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 2009;123:e1-8.



4. Farinha NJ, Razali KA, Holzel H, Morgan G, Novelli VM. Tuberculosis of the central nervous system in children: a 20-year survey. J Infect 2000;41:61-8.


5. Yaramis A, Gurkan F, Elevli M, Soker M, Haspolat K, Kirbas G, et al. Central nervous system tuberculosis in children: a review of 214 cases. Pediatrics 1998;102:E49.



6. Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 2013;12:999-1010.


7. Mahadevan B, Mahadevan S, Serane VT. Prognostic factors in childhood tuberculous meningitis. J Trop Pediatr 2002;48:362-5.


8. Misra UK, Kalita J, Srivastava M, Mandal SK. Prognosis of tuberculous meningitis: a multivariate analysis. J Neurol Sci 1996;137:57-61.


9. Rohlwink UK, Donald K, Gavine B, Padayachy L, Wilmshurst JM, Fieggen GA, et al. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. Dev Med Child Neurol 2016;58:461-8.




10. Dhawan SR, Gupta A, Singhi P, Sankhyan N, Malhi P, Khandelwal N. Predictors of neurological outcome of tuberculous meningitis in childhood: a prospective cohort study from a developing country. J Child Neurol 2016;31:1622-7.



11. Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2014;14:947-57.


12. Fatema K, Rahman MM, Akhter S, Akter N, Paul BC, Begum S, et al. Clinicoradiologic profile and outcome of children with tubercular meningitis in a tertiary care hospital in Bangladesh. J Child Neurol 2020;35:195-201.



13. Israni AV, Dave DA, Mandal A, Singh A, Sahi PK, Das RR, et al. Tubercular meningitis in children: clinical, pathological, and radiological profile and factors associated with mortality. J Neurosci Rural Pract 2016;7:400-4.



14. Bang ND, Caws M, Truc TT, Duong TN, Dung NH, Ha DT, et al. Clinical presentations, diagnosis, mortality and prognostic markers of tuberculous meningitis in Vietnamese children: a prospective descriptive study. BMC Infect Dis 2016;16:573.




15. Ramzan A, Nayil K, Asimi R, Wani A, Makhdoomi R, Jain A. Childhood tubercular meningitis: an institutional experience and analysis of predictors of outcome. Pediatr Neurol 2013;48:30-5.


16. Gupta R, Kushwaha S, Thakur R, Jalan N, Rawat P, Gupta P, et al. Predictors of adverse outcome in patients of tuberculous meningitis in a multi-centric study from India. Indian J Tuberc 2017;64:296-301.


17. Nataprawira HM, Ruslianti V, Solek P, Hawani D, Milanti M, Anggraeni R, et al. Outcome of tuberculous meningitis in children: the first comprehensive retrospective cohort study in Indonesia. Int J Tuberc Lung Dis 2016;20:909-14.


18. van Toorn R, Schaaf HS, Laubscher JA, van Elsland SL, Donald PR, Schoeman JF. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J 2014;33:248-52.


19. Springer P, Swanevelder S, van Toorn R, van Rensburg AJ, Schoeman J. Cerebral infarction and neurodevelopmental outcome in childhood tuberculous meningitis. Eur J Paediatr Neurol 2009;13:343-9.


20. Schoeman J, Wait J, Burger M, van Zyl F, Fertig G, van Rensburg AJ, et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol 2002;44:522-6.


21. Wait JW, Schoeman JF. Behaviour profiles after tuberculous meningitis. J Trop Pediatr 2010;56:166-71.


22. Marais S, Thwaites G, Schoeman JF, Torok ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010;10:803-12.


23. Patkar D, Narang J, Yanamandala R, Lawande M, Shah GV. Central nervous system tuberculosis: pathophysiology and imaging findings. Neuroimaging Clin N Am 2012;22:677-705.


24. Nabukeera-Barungi N, Wilmshurst J, Rudzani M, Nuttall J. Presentation and outcome of tuberculous meningitis among children: experiences from a tertiary children’s hospital. Afr Health Sci 2014;14:143-9.



25. Patel NH, Jain AR, Iyer VK, Shah AG, Jain DA, Shah AA. Clinico-diagnostic and therapeutic relevance of computed tomography scan of brain in children with partial seizures. Ann Indian Acad Neurol 2013;16:352-6.



26. Wasay M, Farooq S, Khowaja ZA, Bawa ZA, Ali SM, Awan S, et al. Cerebral infarction and tuberculoma in central nervous system tuberculosis: frequency and prognostic implications. J Neurol Neurosurg Psychiatry 2014;85:1260-4.


27. Alarcon F, Duenas G, Cevallos N, Lees AJ. Movement disorders in 30 patients with tuberculous meningitis. Mov Disord 2000;15:561-9.


28. Leiguarda R, Berthier M, Starkstein S, Nogues M, Lylyk P. Ischemic infarction in 25 children with tuberculous meningitis. Stroke 1988;19:200-4.


29. Obeso JA, Gimenez-Roldan S. Clinicopathological correlation in symptomatic dystonia. Adv Neurol 1988;50:113-22.

30. Wu XR, Yin QQ, Jiao AX, Xu BP, Sun L, Jiao WW, et al. Pediatric tuberculosis at Beijing Children’s Hospital: 2002-2010. Pediatrics 2012;130:e1433-40.



31. Karande S, Gupta V, Kulkarni M, Joshi A. Prognostic clinical variables in childhood tuberculous meningitis: an experience from Mumbai, India. Neurol India 2005;53:191-6.


32. Mihailidou E, Goutaki M, Nanou A, Tsiatsiou O, Kavaliotis J. Tuberculous meningitis in Greek children. Scand J Infect Dis 2012;44:337-43.


33. Solomons RS, Visser DH, Donald PR, Marais BJ, Schoeman JF, van Furth AM. The diagnostic value of cerebrospinal fluid chemistry results in childhood tuberculous meningitis. Childs Nerv Syst 2015;31:1335-40.



34. Solomons RS, Visser DH, Friedrich SO, Diacon AH, Hoek KG, Marais BJ, et al. Improved diagnosis of childhood tuberculous meningitis using more than one nucleic acid amplification test. Int J Tuberc Lung Dis 2015;19:74-80.


35. Bhatia R, Dayal R, Jindal S, Agarwal D, Goyal A. GeneXpert for diagnosis of tubercular meningitis. Indian J Pediatr 2016;83:1353-5.



36. Pai M, Flores LL, Pai N, Hubbard A, Riley LW, Colford JM Jr. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2003;3:633-43.


37. Scheld WM, Whitley RJ, Marra CM. Infections of the central nervous system. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
38. Padayatchi N, Bamber S, Dawood H, Bobat R. Multidrug-resistant tuberculous meningitis in children in Durban, South Africa. Pediatr Infect Dis J 2006;25:147-50.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,902 View
- 77 Download
- Related articles in Ann Child Neurol
-
Study of Priority between Prognostic Factors of Status Epilepticus in Childhood.2005 May;13(1)






