 |
 |
- Search
| Ann Child Neurol > Volume 30(4); 2022 > Article |
|
Abstract
Purpose
In the past, the use of medium-chain triglycerides (MCTs) for a ketogenic diet (KD) was expected to improve both patientsŌĆÖ and caregiversŌĆÖ adherence to treatment, but many gastrointestinal problems have been reported. Through a calculated partial administration of MCTs in a KD, we aimed to reduce these complications, while maintaining acceptable seizure reduction.
Methods
At a tertiary referral center for pediatric patients with epilepsy, MCT oil was given in a 1:1 ratio with long-chain triglycerides to patients on KDs. Patients who began the diet from February 2019 to February 2020 were reviewed retrospectively, and 47 patients with at least 3 months of follow-up records were enrolled in the study
Results
Overall, 29.8% of patients on a KD with an adjusted MCT ratio experienced complications, such as gastrointestinal symptoms and behavioral food refusal, compared to 63.0% of prior KD patients. The mean seizure reduction rate was 68.45%┬▒40.61%, which was not significantly different from the comparison groupŌĆÖs rate of 64.84%┬▒34.24%.
The ketogenic diet (KD) has been widely recognized as one of the most effective therapies for drug-resistant epilepsy in the pediatric population [1,2] since its introduction by Wilder [3]. The KD, which involves a restricted diet of high fat and low carbohydrates, is expected to increase ketone body concentrations, which can enhance inhibitory neurotransmission and thereby control seizures to some extent [4]. Several studies have shown that KD therapy is effective for treating drug-resistant epilepsy and metabolic disorders such as glucose transporter 1 (GLUT1) de’¼üciency [5] and pyruvate dehydrogenase de’¼üciency [6] in children and adolescents. Several randomized controlled trials have shown that dietary treatment can reduce seizure frequency by half in 38% to 72% of patients with 3 months of therapy [2,4,7].
There are several types of KDs, with the classical KD comprising fat in combination with carbohydrates and protein in specific ratios, ranging from 2:1 to 5:1, and typically utilizing long-chain triglycerides (LCTs) for fat [8]. Adherence to restricted food choices and amounts must be strictly enforced in order to maintain the targeted ketone range, and even minor lapses, such as sugar-containing syrup medication, can compromise treatment efficacy. These dietary restrictions can be challenging for both patients and caregivers, especially for children whose adherence may be inconsistent. Food refusal, limited daily intake, and behavioral problems (e.g., anger and non-acceptance) are common manifestations of adherence problems [9]. Various types of KDs have been designed to improve adherence without compromising the seizure control effects: the modified KD, modified Atkins diet (MAD), low glycemic index treatment (LGIT), and medium-chain triglyceride diet (MCD) [10].
Medium-chain triglycerides (MCTs), such as triglycerides (TG) of octanoic and decanoic acids, are more ketogenic than LCTs, as they lead to the generation of more ketones per kilocalorie of energy. By replacing LCTs in a KD, MCTs can allow more carbohydrate and protein consumption with less total fat, resulting in more acceptable meals and snacks for patients [11]. Huttenlocher et al. [11] first used MCTs for a KD with the expectation of providing a more acceptable diet, and their study in 1971 found that the MCD showed similar seizure reduction rates to the classic KD. Other studies by Schwartz et al. [12] and Neal et al. [13] found no significant difference between the classic KD and MCD when using MCTs with a KD. However, gastrointestinal (GI) problems such as vomiting, bloating, diarrhea, and abdominal pain were common, and MCTs have not been widely used in KDs despite their non-inferior efficacy in seizure control [11-13].
If this high incidence of GI side effects can be offset by modifying the previously used MCD, the advantages of MCTs over LCTs could be utilized more widely. This may be more beneficial for some patients who need more leniency from the classic KD. Both a greater variety of choices of food and larger amounts of food would be desirable for young patients, allowing more freedom of diet and more materials to support ongoing body growth. In the early MCD studies, MCTs comprised 60% of the dietŌĆÖs total energy, and this amount could have caused the frequently reported GI troubles [11]. Few studies have reported the implementation of MCD and even fewer have discussed any new modifications of the MCD to control the side effects.
After incorporating lower percentages of MCTs than previously reported in other MCD studies, we have observed that patients were able to tolerate the therapeutic diet better than what was expected from past literature reviews. Subsequently, there have been a small number of studies that have tested other versions of MCDs with lower percentages of MCTs and supplemental LCTs. Along with the levels of ketosis, the amount of total fat and MCTs in various forms of food was also taken into consideration, and a mixed-lipid diet (MLD) with a 1:1 ratio of MCTs and LCTs was tried in a number of patients at our center. By comparing them to previous KD patients, we aimed to investigate the tolerability and efficacy of the MCT and LCT mixed diet, which may become a more feasible KD for pediatric epilepsy patients.
Patients from the ages of 6 months to 18 years who initiated a KD at a tertiary referral center between February 2019 and February 2020 were recruited retrospectively by reviewing their medical records, and those who incorporated MCTs into their diet were enrolled in the study. Patients who had been followed for less than 3 months or who had not visited the clinic regularly after starting the diet were excluded.
As the primary endpoint of the study was to assess and analyze the complications of the MCD, patients who started the diet for cognitive or developmental improvement were also included in the study to increase the pool of patients. The small number of recruited MCD patients, with an even smaller number of complications, may have resulted in incomparable analyses.
A cohort of patients on a KD without MCTs was selected from the patients treated at the same tertiary center from 2017 to 2020. PatientsŌĆÖ sex, age at the start of KD, and type of KD were taken into account, and those who had maintained the therapy for less than 3 months were excluded. Patients who were put on the diet for cognitive and developmental reasons were also selected in similar proportions to those of the patients in the MCT diet group.
Patients on a KD with MCTs began with one of the various KDs: 4:1, 3:1, 2:1 ratio, MAD, and LGIT. Only after the full implementation of one of these diets were MCTs introduced into the diet, starting with 5% of the total calories. During admission, the amount of MCTs was gradually increased by 5% of the total calories, usually over a 1- to 2-day period. The target MCT quantity was 50% of the total fat amount for the designated KD, with the remaining fat being LCT oil, resulting in a 1:1 ratio of MCTs in quantity. However, the serving amount was limited to 14 g per meal and 52 g per day to minimize potential side effects, and the lower-ketogenic-ratio diets allowed a wider range of food into the diet, reducing the necessary amount of supplementary oil and the quantity of MCT oil, therefore resulting in a lower amount of MCT oil than the target. When the calories from each type of oil were calculated, the aim was for MCTs to provide approximately 25% to 30% of the total fat calories, and about 20% to 25% of the dietŌĆÖs total calories. Supplemental vitamins and minerals were fully provided.
If any complications pertaining to the diet arose, such as abdominal pain, vomiting, diarrhea, or other GI symptoms, the amount of MCTs was usually reduced by 10%.
According to the centerŌĆÖs KD protocol, the majority of patients were admitted and evaluated at the start of KD. Patients were regularly followed up at the outpatient clinic after successful diet induction. The admission, hospital stay, and clinic medical charts were reviewed, and patientsŌĆÖ epilepsy status and general condition were evaluated at baseline during admission, as well as at 1, 3, 6, and 9 months at the outpatient clinic.
The primary endpoint of this study was the occurrence of side effects while patients were on the MCT-including KD, including clinical symptoms and laboratory results. Any related findings were categorized as early or late complications based on the time since the start of the KD. Tolerability was determined by the responses of parents or caregivers.
The secondary endpoint of this study was to assess the change in the seizure rate when compared to the baseline seizure frequency at diet initiation, in order to establish whether the MCD had non-inferior efficacy for epilepsy. This was assessed by parental or caregiver seizure records. Another secondary endpoint was any unexpected abnormal laboratory findings regarding the incorporation of MCTs into the KD. Total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and TG levels were compiled. These laboratory results were also compared from baseline to 1, 3 months, and if possible, also for 6 and 9 months. Withdrawals from the dietary treatment at any period were documented, and the reasons for withdrawal were also reviewed.
SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. To compare the basic characteristics and seizure outcomes of the MCT group and its comparative cohort group, the Student t-test, chi-square test, Fisher exact test, and Mann-Whitney U test were used. The Fisher exact test was used to compare complications between the two groups, and the Student t-test and, primarily, the Mann-Whitney test were used to analyze the lipid profiles of the two groups. The threshold for statistical significance was set at P=0.05.
Fig. 1 depicts how the patients were recruited and grouped for this study. From February 2019 to February 2020, 65 patients on the KD had MCT oil added to their diet, and 49 patients were able to stay on the diet for at least 60 days after starting. In total, 47 patients continued the KD with MCT oil for at least 3 months, 37 for more than 6 months, and 18 for 9 months.
The study patientsŌĆÖ clinical characteristics are summarized in Table 1. The group was made up of more boys (61.7%) than girls, and the patients ranged in age from 12.4 to 197.6 months. About half of them experienced daily seizures, and the patients with seizures were prescribed an average of 2.47┬▒1.53 anti-seizure medications. Several etiologies were suspected as the causes of the patientsŌĆÖ seizures, mainly genetic conditions such as Dravet syndrome, GLUT1 deficiency syndrome, or alterations of genes such as CDKL5, CSTB, DNM1, GRIN1, and KCNQ2. Other etiologies included epilepsy with metabolic causes and structural etiologies such as focal brain lesions, while many patients had undetermined etiologies. It was difficult to match the comparative cohort group in terms of a similar variety of etiologies; however, the two groups showed no significant differences in the etiologies of epilepsy.
Twenty-six patients started with the MAD, 16 patients with a 3:1-ratio KD, and one patient each received LGIT, a 2:1-ratio KD, and a 4: 1-ratio KD. During the 9-month follow-up period, 10 patients adjusted their diet ratio: five for better tolerability and five for better seizure control or cognitive improvement. The use of MCT oil was maintained during these adjustments.
To evaluate short-term seizure outcomes, changes in the seizure rate were measured by comparing the seizure frequency from each time interval to the number of seizures at baseline. As four patients were put on the diet with the expectation of improving their cognitive development, they were excluded from the seizure outcome analysis. At the 3-month follow-up visit, 27 patients (27/43, 62.8%) had more than 90% seizure reduction, and 22 patients (22/43, 51.2%) were seizure-free. Altogether, 86.0% of the patients (37/43) showed a 50% or greater reduction in seizure frequency at the 3-month follow-up visit. Six months after starting the diet with MCT oil, 37 patients maintained the diet, and 21 patients (21/37, 56.8%) were seizure-free. Furthermore, 70.3% (26/37) were able to reduce their baseline seizure frequency by 90% or more. Thirty-one patients (31/37, 83.8%) had their seizures reduced by 50% or more at the 6-month follow-up interval. At the 9-month interval, corresponding to the last follow-up visit for the study, 18 patients remained, of whom 11 (11/18, 61.1%) had achieved seizure freedom. Twelve patients were able to reduce their baseline seizures by 90% and 16 by 50% or higher.
At the 3- and 6-month follow-ups, the MLD and comparative cohort group showed statistically significant differences in the median values of the seizure reduction rate (100.00%, 50.00%-100.00% vs. 75.00%, 50.00%-99.00%, P=0.041 at 3 months; and 100.00%, 50.00%-100.00% vs. 66.66%, 50.00%-91.76%, P=0.027 at 6 months). However, at the last follow-up (after 9 months), the two groups showed no significant difference in the median seizure reduction rate (100.00%, 72.50%-100.00% vs. 90.00%, 61.67%-100.00%, P=0.403). The overall average seizure reduction rate at the last follow-up for patients who were treated for a minimum of 3 months was 68.45%┬▒40.61% in the MLD group and 64.84%┬▒34.24% after treatment for a median duration of 6 months in both groups (Table 2). The 43 patients who maintained the KD with MCT oil for at least 3 months were prescribed an average of 2.47┬▒1.53 anti-seizure medications at the time of diet initiation. While 17 (39.5%) of them were able to reduce the number of medications or lower the dosage of the medications, the mean number of anti-seizure medications after MLD was 2.42┬▒1.71, with no significant difference between before and after the diet (P=0.90). The other KD group also showed no statistically significant change in the mean number of prescribed medications (P=0.92).
Early complications of dietary therapy occurred in 14 patients during diet implementation or within the first month after diet initiation, with 19 total cases of insufficient oral intake, dehydration, metabolic acidosis, vomiting, or abnormal laboratory results. Following the first month of the diet, three patients experienced weight loss, frequent vomiting, and hypercalciuria, which were recorded as late complications (Table 3). In contrast, 63.0% of the patients in the comparative cohort group (17/27) reported a total of 25 cases of KD-related problems. Vomiting (n=3), poor oral intake (n=2), food refusal (n=2), and single cases of diarrhea, abdominal discomfort, constipation, and several abnormal laboratory findings (n=9) were noted during the first month after KD induction, and similar cases of GI symptoms and laboratory results were reported, albeit with a lower incidence, during the latter part of KD (Table 3).
In a comparison of the number of reported troubles due to the KD between the MLD group and the non-MLD group, no significant differences were found in any categories in both the early and late stages of the dietary treatment. Taking all the complications into account, the patients from the MLD group reported fewer complications in the later stage of the diet, constituting a significant difference from the comparative group (P=0.014). Overall, fewer patients experienced KD-related complications in the MLD group during the entire KD process than in the non-MLD (P=0.005).
These complications, in both the early and later stages of the dietary treatment, contributed to termination of the diet in some patients. Thirteen patients (13/47, 27.7%) discontinued the MCT oil diet during the 3- to 9-month follow-up period; while five of them had suffered from the abovementioned complications, eight patients stopped the diet due to poorer than expected seizure control and either added anti-seizure medications or considered other treatment modalities, such as corpus callosotomy or vagus nerve stimulation. In the comparative cohort group, 16 patients discontinued the KD, half of whom did so due to poor seizure control. Four other patients could not continue the diet because of GI symptoms such as vomiting or abdominal discomfort, two patients due to poor oral intake, and two patients due to the caretakerŌĆÖs poor adherence to the diet regimen.
Laboratory results from baseline and follow-up visits of the patients in both groups were collected and statistically analyzed (Table 4). Mean values were obtained from the remaining patients at each follow-up period. While total cholesterol, HDL-C, LDL-C, and non-HDL-C levels all increased during the KD, there was no significant difference between the two groups from baseline to 1 and 3 months. When compared to the MCT oil group, TG levels in the comparative cohort group increased significantly faster at 3 months.
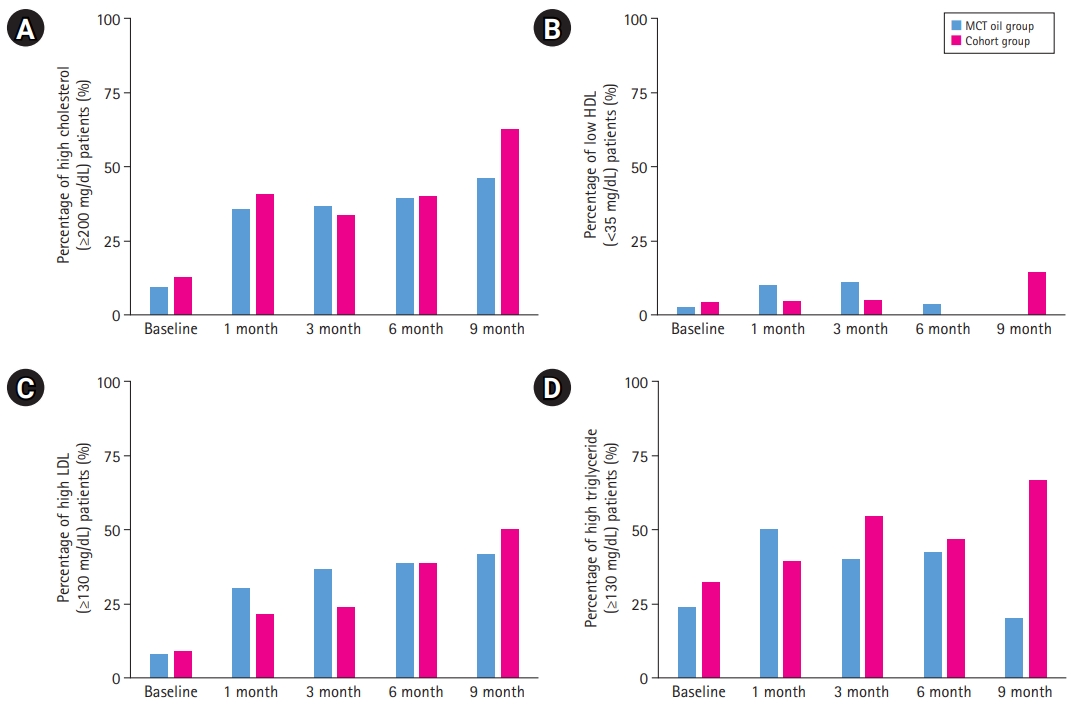
According to the pediatric panel of the National Cholesterol Education Program (NCEP) in the United States, each component of the lipid profile can be categorized into high, borderline, and low levels, and the lipid results of the patients in both groups were analyzed in those terms (Fig. 2). Only 7.7% of the MCT-oil-added patients and 8.7% of the patients in the comparative cohort group had high LDL levels (above 130 mg/dL) at the start of the diet, and this proportion increased throughout the treatment period, ending up with 41.7% and 50% of respective groups having high LDL levels. At baseline, 9.5% and 12.5% of both groups had total cholesterol levels above the acceptable range (200 mg/dL), respectively, and after 9 months of the KD, 46.2% of the MCT-oil group and 62.5% of the comparative cohort group had high total cholesterol levels. Acceptable HDL-C levels (>45 mg/dL) were found in 68.3% of the patients in the MCT oil group and in 75.0% of the comparative cohort group at baseline; however, this higher percentage in the comparative cohort group changed after 9 months, when the corresponding proportions were 91.7% and 57.1%, respectively.
The NCEP pediatric panel recommends different acceptable TG ranges for children aged 0 to 9 and children aged 10 and up; because the study group mostly comprised children under the age of 10 (40 out of 43), only TG levels in this specific range group were compared. After baseline, 23.7% of patients in the MCT-oil group and 32.0% of those in the comparative cohort group had elevated TG levels (100 mg/dL); by 9 months after KD initiation, these proportions changed to 20.0% and 66.7%, respectively.
As the history of MCT oil incorporation into KDs is not short, some previous studies have evaluated its efficacy and clinical applications. Neal et al. [13] conducted a randomized controlled trial demonstrating that the classical KD is not superior to an MCT-based diet in terms of efficacy and tolerability. A prospective long-term study (2 years of follow-up) on KD patients, 79.2% of whom were supplemented with MCT oil, also showed good tolerability and non-inferior efficacy of the diet [14]. However, considering the ability of MCT oil to facilitate relaxation of dietary restrictions, the benefits of using MCT oil over other LCTs were not as prominent as expected. By fine-tuning the MCT oil application in KD, with a 1:1 ratio between MCT oil and LCTs, we aimed to optimize the patientsŌĆÖ KD experience with fewer adverse effects and comparable seizure control.
GI dysfunction such as vomiting and abdominal pain or discomfort has previously been noted as a major problem in maintaining the KD, and this issue was also present in this studyŌĆÖs patients. When compared to past MCT oil studies for KD treatment, the percentage of patients with vomiting (4.3%) or abdominal discomfort (4.3%) was lower. Complaints from patients or caretakers of decreased food intake or behavioral food refusal, as well as laboratory results depicting dehydration or metabolic acidosis, may also possibly be due to GI problems and should be included as such, but this specification of the side effects was consistently applied in previous works and our comparative cohort group. The slightly lower MCT ratio (20% to 25% of the dietŌĆÖs total calories) in this study could be a reason for the lower frequency of GI complications.
In patients consuming a KD, the lipid profile shows increases in total cholesterol, LDL-C, non-HDL-C, and TG levels throughout the diet period; this pattern was observed in both groups in our study. The proportions of patients with high levels of total cholesterol, LDL-C, and TG, and as well as that of patients with low HDL-C levels, increased much more sharply in the cohort group. This may be in line with a previous observation of less dyslipidemia with the use of MCTs for a KD than with the classical KD [15].
These advantages of utilizing MCT oil for a KD may explain the lower dropout rate before 3 months (6.4%) and the overall period (29.8%), than reported in prior studies and observed in this studyŌĆÖs comparative cohort (59.3%). Fortunately, this advantage did not seem to come at the cost of efficacy in seizure control, as this study showed comparable seizure reductions in all follow-up periods (median seizure reduction rates of 100.00% [50.00%-100.00%] at 3 months, 100.00% [50.00%-100.00] at 6 months, and 100.00% [72.50%-100.00%] at 9 months of follow-up). These results are higher than those reported by previous studies, with 79.1% of patients showing more than 50% seizure reduction at their respective final follow-up visits [16].
These favorable findings for the mixed MCT and LCT KD may have arisen from the uncontrolled selection of patients. This study enrolled a large number of patients with GLUT1 deficiency syndrome (14.9%), and Dravet syndrome (10.6%), who are known to benefit from a KD. The low percentage of patients with focal seizures, which are associated with a less favorable response to KD, may also have played a role in these results [17]. This, along with the small sample size, is the main limitation of this study, and it would be premature to apply these findings to all KD programs. A prospective study with a longer follow-up term is required to confirm these advantages in utilizing MCT oil for KDs.
In conclusion, while KD is now an accepted treatment option for patients with intractable epilepsy, it can still be challenging for many patients and families. Aside from adjusting the ratios of the KD, utilizing MCT oil as an alternative source of fat can be just as effective, despite near-failures or complications in the past. This study demonstrated the possibility of a more tolerable, and thus sustainable, solution for dietary therapy by designing a KD with a 1:1 ratio of MCT and LCT oil.
Conflicts of interest
Hoon-Chul Kang is an associate editor, Joon Soo Lee and Heung Dong Kim are editorial board members of the journal, but They was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Notes
Author contribution
Conceptualizations: HDK. Data Curation: RY and EJL. Formal analysis: RY and HCK. Methodology: HDK. Project administration: RY and EJL. Writing-original draft: RY. Writing-review & editing: RY, EJL, JSL, HCK, and HDK.
Fig.┬Ā2.
Percentage of high levels of lipid profile at baseline, and after 1, 3, 6, and 9 months of ketogenic diet. (A) Comparison of percentage of high total cholesterol (Ōēź200 mg/dL) patients. (B) Comparison of percentage of low high-density lipoprotein cholesterol (HDL-C) (<35 mg/dL) patients. (C) Comparison of percentage of high low-density lipoprotein cholesterol (LDL-C) (Ōēź130 mg/dL) patients. (D) Comparison of percentage of high triglyceride (Ōēź100 mg/dL) patients. MCT, medium-chain triglyceride.
Table┬Ā1.
Characteristics of patients who received a ketogenic diet with MCT oil and the comparative cohort group
| Characteristic | MCT oil group (n=47) | Comparative cohort (n=27) | P value |
|---|---|---|---|
| Age at initiation of the ketogenic diet (yr) | |||
| ŌĆā0-Ōēż3 | 18 | 11 | 0.385a |
| ŌĆā3-Ōēż7 | 20 | 7 | |
| ŌĆā7-Ōēż10 | 6 | 7 | |
| ŌĆā<10 | 3 | 2 | |
| Age (mo) | 59.47 (27.17-81.03) | 51.40 (23.83-93.03) | 0.987b |
| Sex (male:female) | 29:18 | 16:11 | 0.979c |
| Etiology | 0.682a | ||
| ŌĆāDravet syndrome | 5 | 1 | |
| ŌĆāGLUT1 deficiency syndrome | 7 | 1 | |
| ŌĆāGenetic epilepsy | 11 | 9 | |
| ŌĆāMetabolic causes | 3 | 1 | |
| ŌĆāStructural etiology (focal) | 6 | 4 | |
| ŌĆāPeriventricular leukomalacia | 3 | 2 | |
| ŌĆāUnknown etiology | 12 | 9 | |
| No. of ASMs | 2.47┬▒1.53 | 2.48┬▒1.42 | 0.932b |
| No. of seizures at initiation | 43 | 24 | 0.119a |
| ŌĆāDaily | 21 | 17 | |
| ŌĆāWeekly | 5 | 5 | |
| ŌĆāMonthly | 11 | 1 | |
| ŌĆāYearly or less | 6 | 1 |
Table┬Ā2.
Ketogenic diet implementation and comparative seizure outcomes
| Variable | MCT oil group (n=47) | Comparative cohort (n=27) | Value |
|---|---|---|---|
| Type of ketogenic diet | |||
| ŌĆā4:1 | 1 | 1 | |
| ŌĆā3:1 | 17 | 7 | 0.865a |
| ŌĆā2:1 | 1 | 1 | |
| ŌĆā1.5:1 | 1 | 0 | |
| ŌĆāModified Atkins diet | 26 | 18 | |
| ŌĆāLow glycemic index treatment | 1 | 0 | |
| Follow-up KD period (mo) | |||
| ŌĆāŌēź3 | 47 | 27 | |
| ŌĆāŌēź6 | 38 | 20 | Žć2=0.465, 0.495c |
| ŌĆāŌēź9 | 18 | 13 | Žć2=0.684, 0.408c |
| Seizure outcomes | 43 | 27 | |
| ŌĆāSeizure reduction rate | 68.45┬▒40.61 | 64.84┬▒34.24 | 0.352b |
| ŌĆāSeizure freedom | 21 (48.8) | 8 (29.6) | Žć2=2.522, P=0.112c |
| ŌĆāŌĆāŌēź90% reduction | 26 (60.5) | 10 (37.0) | Žć2=3.644, P=0.056c |
| ŌĆāŌĆāŌēź50% reduction | 34 (79.1) | 23 (85.2) | P=0.753a |
Table┬Ā3.
Early and late complications of ketogenic diets in the two groups
| Types of complications |
MCT oil group (n=47) |
Comparative cohort (n=27) |
P value |
||||
|---|---|---|---|---|---|---|---|
| Early (start-1 mo) | Late (>1 mo) | Early (start-1 mo) | Late (>1 mo) | Early | Late | All | |
| Gastrointestinal symptoms | 2 | 1 | 6 | 4 | 0.045a | 0.056 | 0.008a |
| ŌĆāVomiting | 2 | 1 | 3 | 2 | |||
| ŌĆāDiarrhea | 0 | 0 | 1 | 1 | |||
| ŌĆāAbdominal discomfort | 2 | 0 | 1 | 0 | |||
| ŌĆāConstipation | 0 | 0 | 1 | 1 | |||
| Poor oral intake | 7 | 0 | 2 | 1 | 0.472 | 0.365 | 0.738 |
| Behavioral food refusal | 0 | 0 | 2 | 0 | 0.130 | 0.130 | |
| Abnormal laboratory results | |||||||
| ŌĆāHypertriglyceridemia | 0 | 0 | 2 | 0 | |||
| ŌĆāLiver enzyme increase | 1 | 1 | 1 | 1 | |||
| ŌĆāAcidosis | 3 | 0 | 4 | 0 | |||
| ŌĆāDehydration | 4 | 0 | 2 | 0 | |||
| ŌĆāHypercalciuria | 0 | 1 | 0 | 2 | |||
| Total | 19 | 3 | 17 | 8 | |||
| Total patients | 14 | 3 | 15 | 8 | 0.047a | 0.014a | |
| Overall cases | 20 | 25 | |||||
| Overall patients | 14 patients (29.8%) | 17 patients (63.0%) | 0.005a | ||||
Table┬Ā4.
Plasma levels of components of the lipid profile at baseline, and after 1 and 3 months of ketogenic diet therapy
| Plasma level | MCT oil group | Comparative cohort | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | |
| Total cholesterol (mg/dL) | 151.7┬▒32.1 (44) | 181.0 (44) (151.0-217.3) | 185.0 (43) (151.0-225.0) | 164.0┬▒27.5 (27) | 189.0 (25) (159.5-233.0) | 180.5 (24) (150.8-218.0) | 0.103a | 0.537b | 0.880b |
| HDL-C (mg/dL) | 50.0 (43) (43.0-57.0) | 51.0 (43) (41.0-62.0) | 52.0 (41) (40.0-58.0) | 51.0 (27) (45.0-59.0) | 55.0 (25) (45.5-65.5) | 50.5 (24) (42.3-66.0) | 0.595b | 0.288b | 0.422b |
| LDL-C (mg/dL) | 84.4┬▒28.3 (40) | 115.6 (39) (79.8-149.4) | 114.0 (34) (85.6-146.9) | 92.9┬▒26.5 (26) | 114.0 (22) (82.2-130.0) | 97.4 (20) (77.5-127.8) | 0.225a | 0.946b | 0.474b |
| Non-HDL-C (mg/dL) | 95.5 (40) (78.3-123.5) | 134.5 (40) (111.3-169.8) | 140.0 (37) (102.5-177.0) | 103.0 (26) (89.8-122.3) | 133.0 (24) (99.0-160.5) | 118.0 (23) (94.0-156.0) | 0.276b | 0.637b | 0.438b |
| Triglycerides | 71.0 (43) (61.0-101.0) | 92.0 (43) (71.0-133.0) | 82.0 (41) (61.0-144.5) | 81.0 (27) (56.0-105.0) | 90.0 (25) (82.0-111.5) | 110.0 (24) (68.0-166.0) | 0.952b | 0.949b | 0.146b |
References
1. Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018;3:175-92.




2. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008;7:500-6.


3. Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Proc 1921;2:307-8.
4. Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios: comparison of 3:1 with 4:1 diet. Epilepsia 2007;48:801-5.


5. Klepper J, Leiendecker B. GLUT1 deficiency syndrome: 2007 update. Dev Med Child Neurol 2007;49:707-16.


6. Sofou K, Dahlin M, Hallbook T, Lindefeldt M, Viggedal G, Darin N. Ketogenic diet in pyruvate dehydrogenase complex deficiency: short- and long-term outcomes. J Inherit Metab Dis 2017;40:237-45.




7. Martin-McGill KJ, Jackson CF, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 2018;11:CD001903.


9. van der Louw E, Olieman J, Poley MJ, Wesstein T, Vehmeijer F, Catsman-Berrevoets C, et al. Outpatient initiation of the ketogenic diet in children with pharmacoresistant epilepsy: an effectiveness, safety and economic perspective. Eur J Paediatr Neurol 2019;23:740-8.


10. Blackford R. Not your parentsŌĆÖ ketogenic diet: flexibility in 2020. Epilepsy Res 2020;162:106307.


11. Huttenlocher PR, Wilbourn AJ, Signore JM. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971;21:1097-103.


12. Schwartz RH, Eaton J, Bower BD, Aynsley-Green A. Ketogenic diets in the treatment of epilepsy: short-term clinical effects. Dev Med Child Neurol 1989;31:145-51.


13. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009;50:1109-17.


14. Lambrechts DA, de Kinderen RJ, Vles JS, de Louw AJ, Aldenkamp AP, Majoie HJ. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol Scand 2017;135:231-9.



15. Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 2003;290:912-20.


- TOOLS








