Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) is a rare neuro-inflammatory disorder with only 10 pediatric cases reported worldwide. The diagnostic criteria proposed in 2017 [
1] require clinical criteria (pontocerebellar dysfunction with an exquisite response to steroids, in the absence of peripheral neurological disease and other explainable etiology) and radiological criteria (homogenous gadolinium-enhancing nodules of less than 3 mm, predominantly in the perivascular regions of pons and cerebellum, which resolve with steroid therapy) to be fulfilled for probable CLIPPERS and additional histopathological criteria (lymphocytic, predominantly T-cells, infiltrating perivascular sites) for definite CLIPPERS. In 2019, revised criteria [
2] that included a change in the size of the nodules to less than 9 mm, were proposed to improve diagnostic specificity. CLIPPERS has different outcomes between children and adults, as at least 30% of pediatric cases were associated with alternative diagnoses such as familial hemophagocytic lymphohistiocytosis (fHLH) and 20% had residual severe disabilities [
2,
3].
We report a case of probable pediatric CLIPPERS with a good outcome in a patient who presented with acute cerebellar signs and classical post-gadolinium brain magnetic resonance imaging (MRI) features of punctate lesions of the hindbrain and responded exquisitely to steroids. He was also screened for fHLH, and no known genetic mutations were detected.
A previously well 10-year-old boy presented with a 1-week history of progressive headache, diplopia, and unsteady gait, with no other neurological or systemic symptoms. There was a history of pancytopenia attributed to viral illness 3 years ago that had self-resolved. On examination, he was afebrile and had a full Glasgow Coma Scale score with appropriate behaviour for his age. He had truncal ataxia and a broad-based gait with past pointing, dysdiadochokinesia, and intentional tremors bilaterally. There was no ophthalmoplegia, fundoscopy showed no optic neuritis or papilledema, all other cranial nerves were intact, and the tone, power, and reflexes of both upper and lower limbs were normal. There was no hepatosplenomegaly or lymphadenopathy, and the findings of other systemic examinations were normal.
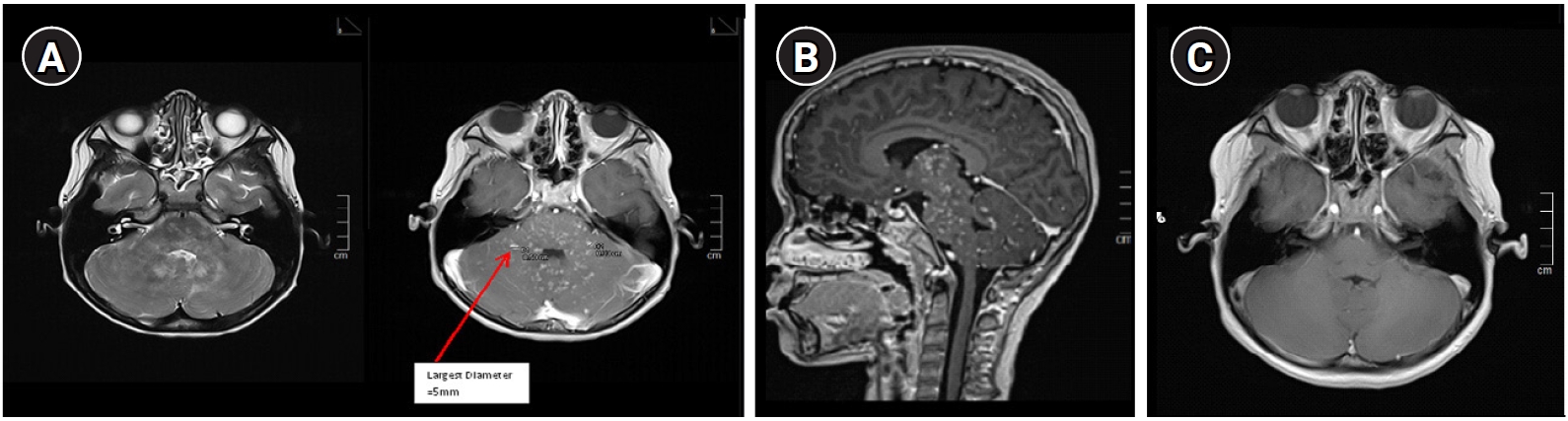
Investigations were conducted with the aim of ruling out infection, posterior circulation stroke, demyelination, or malignancy. His full blood profile had no features of hematological malignancy. Complement levels (C3 and C4) and levels of immunoglobulin G, A, M, and E were within normal limits. Antinuclear antibody and aquaporin-4 antibody were negative. Cerebrospinal fluid (CSF) showed a normal protein level of (0.487 g/L), a glucose ratio of 72%, a cell count of 0, and negative culture findings. CSF oligoclonal bands were not detected. Brain computed tomography showed no mass, hemorrhage, or infarcts. Brain MRI with contrast showed signal abnormalities of punctate and curvilinear-pattern enhancement involving the perivascular space in the cerebellum and pons, with cranial and caudal extension on T2 and post-gadolinium images (
Fig. 1A and
B).
As the patient fulfilled the clinical and radiological diagnostic criteria, he was treated for probable CLIPPERS. He was given intravenous methylprednisolone (30 mg/kg/day) for 5 days followed by oral prednisolone (1 mg/kg/day) for 2 weeks and then a dose of 0.5 mg/kg/day for 1 month. His symptoms started to show marked improvement upon completion of intravenous steroid treatment. A repeated brain MRI 6 weeks post-treatment showed that the lesions had completely resolved (
Fig. 1C). He was gradually weaned from oral steroid therapy over the next 4 months (the total course of oral steroids was nearly 6 months). He remained well at 19 months post-diagnosis with no recurrence clinically and radiologically. He was screened for fHLH, and the genetic panel for immunodeficiency was negative for known fHLH gene mutations. Serum ferritin, triglyceride, lactate dehydrogenase, and fibrinogen levels were normal, as was the full blood profile. However, heterozygous gene mutations corresponding to variants of uncertain significance were identified for
LRRC8A exon 3, c.2023G>A (p.Glu675Lys);
STK4 exon 7, c.823C>G (p.Leu275Val); and
TNFRSF9 exon 8, c.626G>A (p.Arg209His).
CLIPPERS is a new and rare entity that was first described recently in 2010 [
1,
2]. It has been predominantly reported among adults, with about 80% of cases having only mild disabilities (modified Rankin Scale score of 3 or less) [
1,
2,
4]. This differs significantly compared to pediatric cases [
3-
9]. The exact mechanism of CLIPPERS is still unclear. Proposed hypotheses include a possible pre-stage of a malignancy or an atypical presentation of well-characterised neuro-inflammatory diseases such as anti-myelin oligodendrocyte antibody disease [
2]. From our observations, as there is a strong association with fHLH (classified as an inborn error of immunity) and T-cell predominance on histopathology, mechanisms related to immune dysregulation need to be considered.
According to our literature review, there is slight male preponderance, with the age of onset from 18 months to 16 years old. Most cases reported had symptoms for more than 1 month (except for patients 1 and 4) (
Table 1) [
3-
10]. There were no specific CSF biomarkers for diagnosis. When describing brain MRI findings, five reported cases mentioned the presence of typical punctate and nodular lesions (patients 1, 2, 3, 8, and 10), but no previous reports stated the size of the largest punctate lesion in the reports, making a direct comparison of cases challenging.
With regards to treatment, all casesŌĆöeither newly diagnosed or relapsedŌĆöwere pulsed with intravenous methylprednisolone, followed by oral steroids. In the pediatric cohort, steroids were maintained for a shorter period (ranging from 3 to 6 months) due to concerns of other diseases, than in adults (more than 6 months). Among those who continued to have ŌĆ£trueŌĆØ CLIPPERS on re-evaluation at relapse, the second-line steroid-sparing agents used were varied and usually were a combination of oral and intravenous administrations [
3-
6,
10]. Although our case is currently in remission clinically and radiologically without steroids, this could be a ŌĆ£honeymoonŌĆØ period and careful monitoring is required, as relapse or conversion to a non-CLIPPERS diagnosis can occur at any time post-diagnosis [
2].
To date, outcomes among children appear less favourable than those among adults, as only 27.3% (3/11) of cases, including ours, recovered completely with no significant disabilities [
6,
10]. Eighteen percent (2/11) died due to treatment-related complications [
3], 18% (2/11) had relapsing disease with severe disabilities [
4,
5], and 36.6% had alternative diagnoses on relapse (either lymphoma or fHLH) [
3,
7-
9].
In summary, we would like to highlight some points relevant to pediatric CLIPPERS. Firstly, lesions on post-gadolinium imaging with a size that is equal or more than 9 mm are likely to be non-CLIPPERS cases, as highlighted in the revised criteria by Taieb et al. [
2] or may have a poorer prognosis as observed in our literature review. Secondly, all pediatric CLIPPERS patients should undergo genetic testing for inborn errors of immunity at either the first diagnosis or relapse (specifically for fHLH). Thirdly, surveillance contrast brain MRI is recommended at least 6 months or earlier (if there is any clinical recurrence) in the first 2 years post-diagnosis, due to the high conversion rate to non-CLIPPERS diagnoses within this period. Finally, the genetic mutations detected in this patient should be kept for reference as they may prove significant for future research related to CLIPPERS.
Written informed consent to publish was obtained from the patient (IRB approval no.: NMRR ID-22-00026-ZFD [IIR]).
Conflicts of interest
Teik Beng Khoo is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Notes
Author contribution
Conceptualization: VWML. Writing-original draft: VWML. Writing-review & editing: TBK, CZBCD, and KBAL.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Fig.┬Ā1.
Magnetic resonance imaging (MRI) brain axial (A) and sagittal section (B) of the hind brain comparing T2w and post-gadolinium images pre-treatment. The arrow shows that the largest punctate lesion (although coalesce) is 5 mm. Diameter of single lesions are no larger than 3 mm. Contiguous lesions were seen on T2w images which corresponded to ŌĆśsalt and pepperŌĆÖ/speckled appearance on the post-gadolinium MRI. Do note that the area involved on T2 do not exceed the post-gadolinium area of lesions and they are located peri-vascularly and can extend juxtacortically or caudally. (C) MRI brain axial post-gadolinium 6 weeks post-treatment showed complete resolution of the lesions.
Table┬Ā1.
Summary of pediatric CLIPPERS cases reported worldwide
|
No. |
Patient |
Clinical symptoms |
Brain MRI (at presentation)
|
Neuropathology (brain biopsy)
|
Other relevant investigations
|
Treatment |
Outcome |
|
1 |
10 yr, male, Malay (this case) |
Acute cerebellar signs for 1 week |
Bilateral punctate Gd+ lesion of the cerebellum, thalamus, and cervical cord (largest lesion 5 mm) |
Not performed |
CSF: acellular. Normal protein & lactate, viral studies neg; Oligoclonal bands neg; ANA, aquaporin-4 neg; Systemic HLH screening neg; fHLH genetics neg |
IVMP followed by oral pred for 6 months |
Good. mRS 0 |
|
No imaging recurrence 19 months since diagnosis (12 months off steroids) |
|
2 |
3 yr, male, White [3] |
Subacute onset of truncal ataxia, bilateral 6th CN palsy, slurred speech, drooling, tremor, past pointing |
Bilateral punctate Gd+ of dorsal pons, thalamus, cerebellar white matter |
Perivascular lymphocyte and macrophage infiltrates (1 year post-steroid) |
CSF: acellular. Normal protein & lactate, viral studies neg; Oligoclonal bands neg |
IVMP followed by oral pred. AZA at 6 years, natalizumab and IVIG at 16 years |
Good |
|
Multiple relapses from 9 years |
|
Non-ambulatory from 15 years |
|
Natalizumab halted disease |
|
Died at 17 years due to infection |
|
3 |
13 yr, male, White [3] |
6-week progressive history of ataxia, dysarthria |
Bilateral nodular lesion with Gd+ in the brainstem, basal ganglia, posterior limb of internal capsule |
Predominant T-cell and histiocytic populations |
CSF: acellular. Persistently slightly high proteins; Neg viral studies; Oligoclonal bands neg |
Cycles of IVMP followed by oral pred |
Remained symptomatic despite treatment |
|
Metabolic study: neg |
MMF, IVIG, PLEX, infliximab |
Repeated brain biopsy: EBV-driven B-cell lymphoma Treated for lymphoma |
|
Genetic study: neg |
ŃĆĆ |
ŃĆĆ |
|
4 |
14 yr, female, White [3] |
Acute encephalopathy with multiple cranial neuropathies post-viral prodrome |
Diffuse cortical grey-white matter T2 hyperintensities at left cerebellar peduncle and dorsal pons |
Non-specific inflammation |
CSF: acellular. Normal protein, lactate; Neg viral PCR |
IVMP, IVIG then followed by oral pred; Relapses treated with Cyclo, AZA, PLEX |
Frequent relapses despite second-line treatment |
|
Metabolic workup: neg |
Patient died at 16 years |
|
5 |
13 yr, male, White [4] |
Ataxia, dysarthria, diplopia, nystagmus, incoordination, psychomotor slowing, tetraparesis |
Gd+ lesions at the supratentorial level, spinal cord, and brainstem swelling |
Parenchymal and perivascular inflammatory infiltrates with mild focal demyelination |
Oligoclonal bands positive |
Pulsed IVMP followed by rituximab |
Multiple relapses
Severe disability |
|
No underlying demyelinating, inflammatory, neoplastic, or vasculitic processes |
|
6 |
16 yr, male, Pakistani [10] |
1-month history of fever, ataxia, distal lower limbs weakness, horizontal nystagmus, seizure |
Hyperintensity on T2 at cerebellar hemispheres with Gd+ peppering lesion on pons and cerebellum |
Not performed |
CSF: pleocytosis with elevated protein
Inflammatory & neoplastic workup: neg |
IVMP followed by oral pred and monthly IVMP pulse for 2 days, AZA, MTX |
Resolved ataxia & radiological lesions; Recurrent seizures (resolved with monthly IVMP pulse & Lev); Activity of daily living independent |
|
7 |
14 yr, male, unknown ethnicity [6] |
2-month history of 6th and 7th CN palsies with dysarthria, ataxia, incoordination, nystagmus |
Patchy and peppered hyperintensities in the bilateral cerebellum and brainstem (mostly pons) |
Not performed |
No underlying demyelinating, inflammatory, neoplastic, or vasculitic processes |
IVMP followed by oral pred |
Improved clinically and radiologically |
|
8 |
10 yr, female, Latina [5] |
3-month progressive lower limb weakness, ataxia, 6th CN palsy and diplopia, clonus and brisk tendon reflexes |
Nodular hyperintensities in the brainstem, cerebellar peduncle, cervical and thoracic spinal cord |
Perivascular T-lymphocytes, non-specific infiltrates |
CSF: pleocytosis
No underlying demyelinating, inflammatory, neoplastic, or vasculitic processes |
IVMP followed by oral pred, MTX, rituximab |
Clinical and radiological relapses |
|
Cumulative disability |
|
9 |
15 yr, male, Indian [7] |
Ataxia, bilateral persistent esotropia, and diplopia |
Gd+ cerebral and cerebellar white matter T2-hyperintense lesions with involvement of pons and midbrain. |
Inflammatory cell infiltrate in the leptomeningeal space, perivascular area, and parenchyma along blood vessels |
No underlying demyelinating, inflammatory, neoplastic, or vasculitic processes |
IVMP followed by oral pred, MTX |
Symptom-free for 6 months with considerable resolution of lesions on MRI; Defaulted treatment and re-presented with fever and pancytopenia |
|
Was treated for multiphasic ADEM at 6 and 12 years |
Final diagnosis: fHLH |
|
10 |
18 mo, female, Caucasian [8] |
Subacute onset of ataxia, unilateral esotropia, mild encephalopathy, hepatosplenomegaly |
Punctate & linear T2 hyperintensities in the middle cerebellum, pons, and thalami |
Perivascular and parenchymal CD3+ T cell-rich infiltrate with scant CD20+ B-cells and CD68+ histocytes (at relapse) |
CSF: lymphocytosis. Raised neopterin; Normal protein |
IVMP followed by oral pred, MMF and monthly IVIG |
Symptom-free for 6 months then clinical+radiological relapse |
|
Systemic HLH screening: abnormal NK cells degranulation activity; Others normal |
Alternative immunotherapy when steroid side effects but relapse when weaned off pred |
|
BMA: normal |
Suspected fHLH (heterozygous UNC13D pathogenic mutation) |
|
11 |
5 yr, female, Caucasian [9] |
Headache, vomiting, mild ataxia, esotropia, nystagmus, tremor, and dysarthria |
Multifocal contrast enhancing cerebral and cerebellar T2 hyperintensities |
Perivascular inflammatory infiltrates involving grey and white matter & leptomeninges |
CSF: pleocytosis. Mildly raised neopterin |
IVMP followed by oral pred |
Relapse with steroid taper |
|
Repeat on relapse: prominent T-cell infiltrates |
Monthly IVIG |
Disease stabilized on natalizumab but relapsed subsequently and diagnosed as fHLH (PRF mutation) at 10 years |
|
ŃĆĆ |
Cyclo |
ŃĆĆ |
|
ŃĆĆ |
Natalizumab |
ŃĆĆ |
|
ŃĆĆ |
Unrelated HCT |
ŃĆĆ |
References
1. Tobin WO, Guo Y, Krecke KN, Parisi JE, Lucchinetti CF, Pittock SJ, et al. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2017;140:2415-25.


2. Taieb G, Mulero P, Psimaras D, van Oosten BW, Seebach JD, Marignier R, et al. CLIPPERS and its mimics: evaluation of new criteria for the diagnosis of CLIPPERS. J Neurol Neurosurg Psychiatry 2019;90:1027-38.


3. Sa M, Green L, Abdel-Mannan O, Chong W, Jacques T, Clarke A, et al. Is chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) in children the same condition as in adults? Dev Med Child Neurol 2019;61:490-6.


4. Taieb G, Duflos C, Renard D, Audoin B, Kaphan E, Pelletier J, et al. Long-term outcomes of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) in a consecutive series of 12 patients. Arch Neurol 2012;69:847-55.


5. Veerapandiyan A, Chaudhari A, Deo P, Ming X. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS): a pediatric case report with six year follow-up. Mult Scler Relat Disord 2017;17:95-8.


6. Mathias S, Hickman D, Lightner D, Smith C, Baumann R. A pediatric case of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids). Neurology 2015;84(Suppl 14):P6.265.
7. Patel V, Udwadia-Hegde A, Hajirnis O, Nemani T, Pandrowala A, Desai M, et al. A rare neurological presentation of familial Hemophagocytic lymphohistiocytosis. J Pediatr Neurol 2021;19:92-101.

8. Debinski C, Goergen S, McLean C, Buckland ME, Kumar B, Tiller G, et al. Exploring the intersection of isolated-CNS hemophagocytic lymphohistiocytosis and pediatric chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. J Child Neurol 2021;36:935-42.


10. Salam A, Sana F, Nazir R, Saeed MA, Iqbal Y, Ahmad A. CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids): case report with neuroimaging. Pak J Neurol Sci 2014;9:26-8.










