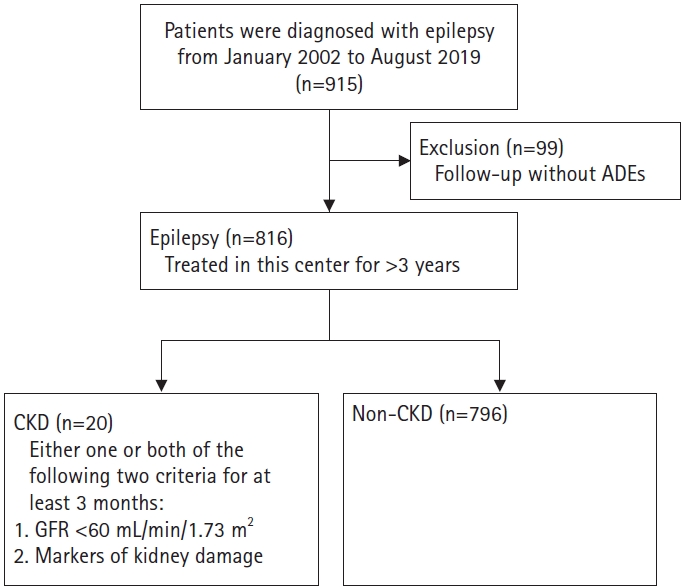
Introduction
Epilepsy is a brain disorder which implies an enduring predisposition to unprovoked seizures [
1,
2]. The main focus of epilepsy treatment is satisfactory seizure control with minimal adverse effects [
3]. Epilepsy treatments in children and adolescents are usually prescribed for at least 2 years (and for life-time in some cases), for which adverse effect control is of utmost importance [
2,
4].
Patients undergoing long-term treatment with antiepileptic drugs (AEDs) may develop other diseases, as well as adverse effects from the drugs, which may limit AED usage [
4,
5]. Several AEDs undergo hepatic metabolism and are excreted via urine. Lamotrigine, phenobarbital, and lacosamide, which are often used in clinical practice, are examples of this pharmacokinetics. On the other hand, levetiramcetam and topiramate remain unchanged before being excreted via urine. Long-term use of these drugs can cause side effects on the kidneys. Some common adverse effects include nephrotoxicity, calciuria, and other events related to the kidneys [
3,
5]. However, and despite the abovementioned, only a few patients with long-term AED treatment develop chronic kidney disease (CKD).
In this paper, we investigated the characteristics of epilepsy patients who developed CKD. Through this study, we aimed to identify risk factors for CKD and analyze which factors should be considered in the treatment plan of patients with these characteristics.
Discussion
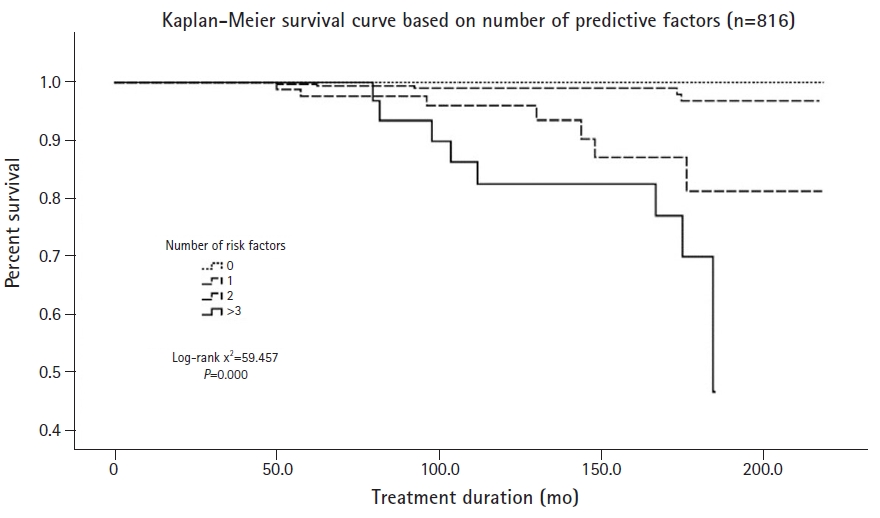
In this study aimed at determining risk factors for CKD in pediatric epilepsy patients, we detected four significant risk factors, namely, initial creatinine, microscopic hematuria, interictal epileptiform discharges, developmental delay.
All patients with ongoing AED treatment underwent urinalysis to detect adverse effects such as nephrotoxicity, hypercalciuria, and nephrolithiasis. A statistical analysis was performed to confirm whether initial hematuria affects kidney function and the multivariate Cox proportional hazards model showed that microscopic hematuria was significantly different between the CKD group (four patients [20.00%]) and the non-CKD group (27 patients [3.39 %]). Microscopic hematuria is a common clinical manifestation in children, adolescents, and young adults, with its incidence ranging from 0.18% to 16.1% according to previous studies [
12-
14]. Hematuria can either be an isolated finding unaccompanied by other urinary abnormalities or be accompanied by urinary abnormalities such as proteinuria, hypertension, and elevated serum creatinine level [
13,
14]. Persistent isolated microscopic hematuria can have either a glomerular or a non-glomerular origin, including several kidney disease entities [
12,
13]. In fact, among the 20 patients in the CKD group, four patients presented glomerulonephritis in the histopathologic examination. The four cases of glomerulonephritis were classified as Henoch-Schonlein nephritis (grade III), focal segmental endocapillary proliferative glomerulonephritis, focal segmental glomerulosclerosis, and minor glomerular change on renal histological examination. Additionally, four patients reported recurring urinary tract infections and nephrolithiasis. The causes of hematuria mentioned above increase the risk of CKD [
14]. In other studies, 0.7% of patients with persistent isolated microscopic hematuria developed end-stage kidney disease compared with 0.04% in the control group, yielding an adjusted HR of 18.5 (95% CI, 12.4 to 27.6) [
14].
The multivariate Cox proportional hazard model result showed that increased serum creatinine levels significantly increased the risk of CKD (HR, 13.927; 95% CI, 1.857 to 104.455;
P=0.010). Because creatinine is excreted from the circulation mainly by renal filtration, it is strongly correlated with GFR [
15-
17]. An increase in serum creatinine reflects an impaired renal ability to remove waste products from the body [
16]. The creatinine concentration increases progressively with decreased GFR, and its diagnostic performance has been shown to be good, even for the detection of minor deteriorations of the renal function [
15,
16]. Hence, creatinine is a strong predictor of CKD progression [
16].
In this study on the prediction of risk factors of CKD, the EEG results were analyzed by classifying them into different findings rather than into a clinical diagnosis. Regarding the presence of interictal epileptiform discharges, the HR of generalized interictal epileptiform discharges was 38.395, and that of focal interictal epileptiform discharges was 19.252 in the CKD group compared to the non-CKD group for initial interictal EEG findings. Patients with interictal epileptiform discharges on EEG tend to have clinical seizure events in contrast to those without. Recurrent clinical seizure events impair cognitive abilities and neurological development. Regarding background activity, when there was a disorganization, the HR was 4.201, indicating borderline significance. Abnormal background activity on the EEG includes a lack of the expected organization corresponding to age, multifocal spikes, and loss of normal physiological hallmarks, like sleep spindles [
18-
20]. These EEG findings are seen in most cases where the brain is structurally and functionally deteriorated, including in LGS, Rett syndrome, and West syndrome. In this study, seven patients with CKD who experienced intractable epilepsy (e.g., LGS, Rett syndrome, West syndrome, Devic syndrome, Wolf-Hirschhorn syndrome) were bedridden [
18-
20]. The comparison was not statistically significant; however, it is possible that abnormal background activity acts as a significant risk factor in larger patient groups considering its borderline significance. In general, only 29% to 55% of epilepsy patients have epileptiform abnormalities on a single routine outpatient EEG. Therefore, even if normal EEG findings are reported in the first examination, abnormal findings often appear in the second and third examinations. However, in the present study we only analyzed the initial EEG results to assess the risk of CKD at the initial planning of diagnosis and treatment.
In addition, 12 (60%) patients in CKD group, including those with intractable epilepsy, had developmental delays. In such cases, the patientsŌĆÖ brain dysfunction may not directly unable to have normal daily lives, it may lead to poor sanitation and urinary retention without voluntary urination [
21]. These patients might be more vulnerable to nephrotoxicity induced by the long-term administration of AEDs. The patientsŌĆÖ decreased voiding function may lead to residual urine. Among these patients, some developed urinary tract stones and recurrent urinary tract infections, which leads to acute kidney injury and affects renal function, and which can contribute to the development of CKD. In the present case, the statistically significant HR related to developmental delay was 11.929.
In conclusion, the risk of CKD increases in cases of epilepsy with high creatinine and microscopic hematuria associated with kidney damage, and with restricted daily activity due to a decreased brain function. In this study on factors associated with an increased risk of CKD, the abovementioned factors had statistically significant results while AED-related factors did not. In the long-term treatment of epilepsy patients, it is important to perform regular kidney function evaluations and to practice regular urine voiding to prevent the development of CKD, in addition to following-up potential AED adverse effects.
This study has some limitations. The study was retrospective in nature, and the sample was not randomized. Moreover, the patients were prescribed different AEDs not based on the medical condition alone, but also for social reasons. In addition, it is possible that factors including AED history, ketogenic diet, and abdominal ultrasonography were not statistically significant owing to the relatively small number of patients in the CKD group. As shown in the study results, AEDs that lead to nephrotoxicity might act as risk factors for patients with vulnerability factors for CKD. Further study is need to validate the risk of CKD generated by AEDs, ketogenic diet, EEG findings, brain function, and kidney factors in larger CKD patient groups.