Trends in Pediatric Meningitis in South Korea during 2009 to 2017: Analysis of the Health Insurance Review and Assessment Service Database
Article information
Abstract
Purpose
Previous estimates of the cause- and age-specific frequency of pediatric meningitis in Korea were mainly derived from single- and multi-center studies. Herein, we used data on the number of cases of bacterial and viral meningitis from the Health Insurance Review and Assessment Service database to examine time- and sex-related trends.
Methods
We extracted data on meningitis diagnoses registered in the Health Insurance Review and Assessment Service from 2009 to 2017, using Korean Standard Classification of Disease and Cause of Death codes. Information on 202,254 children aged 0 to 18 years was extracted. Detailed demographic and disease information was available for 84,543 children who underwent hospitalization.
Results
Among all hospitalized patients, 2166, 36,155, and 46,192 children were diagnosed with bacterial, viral, or other types of meningitis, respectively. There were 30 cases of fungal meningitis and another 30 cases of meningitis attributable to other pathogens. The number of cases of bacterial and viral meningitis was highest among infants (1,087 [50.2%]) and patients in their early childhood (12,949 [35.9%]), respectively. Meningitis outbreaks were most likely to occur during the summer, and boys were more susceptible to meningitis than girls. The following pathogens most commonly caused infant meningitis: group B Streptococcus, Escherichia coli, and type B Haemophilus influenzae.
Conclusion
This study reports the number of pediatric meningitis cases, stratified by age, disease type, and month/year. The present findings contribute to a better understanding of pediatric meningitis in Korea and provide a foundation for future research to identify the risk factors for this disease.
Introduction
Meningitis is a condition in which the meninges surrounding the brain and spinal cord are inflamed [1,2]. Among other infectious diseases that occur in children, meningitis is the most common cause of central nervous system disease [1]. Meningitis is associated with a high risk of systemic infection, which can lead to serious sequelae; thus, careful diagnosis and treatment are paramount [3-5]. It is broadly categorized as bacterial meningitis, viral meningitis, and meningitis due to other cause (e.g., parasites or fungi), and it tends to occur as a result of spread of infection to the meninges from elsewhere in the body [1,6].
Bacterial meningitis requires rapid diagnosis and treatment as it is associated with a high risk of sepsis, which has a mortality rate of 10% [2,7,8]. Viral meningitis is more common than bacterial meningitis and it tends to present as a relatively less severe disease, although it can lead to serious complications and long-term disability [9-12]. Fungal and parasitic meningitis are rare but can progress to severe disease via the mediation of immune suppressors [1,13].
Previous reports on the cause- and age-stratified frequency of pediatric meningitis in Korea have primarily originated from single- and multi-center studies [14,15]. In contrast, the present study used data on the frequency of bacterial and viral meningitis obtained from the Health Insurance Review and Assessment Service (HIRA) database to examine time- and sex-related trends. Recently, various studies have been started in relation to the HIRA database, but there has been no recent prevalence study in children in Korea. Since this study included data across all ages and medical institutions, it can provide an objective and accurate prediction of the prevalence and future trends of current diseases.
Materials and Methods
1. Data sources
The HIRA database is a data repository, built using information communication technology [16], that captures information of claimants under Korea’s National Health Insurance scheme, including details of disease screening, diagnostic evaluation and medical resources required, and socio-economic status. Annually, data from 87,000 medical claims become available for research purposes. Use of these data for this study was approved by the Institutional Review Board of Chung-Ang University Hospital. The study was designed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chung-Ang University (IRB No.: 1921-002-360). Written informed consent by the patients was waived due to a retrospective nature of our study.
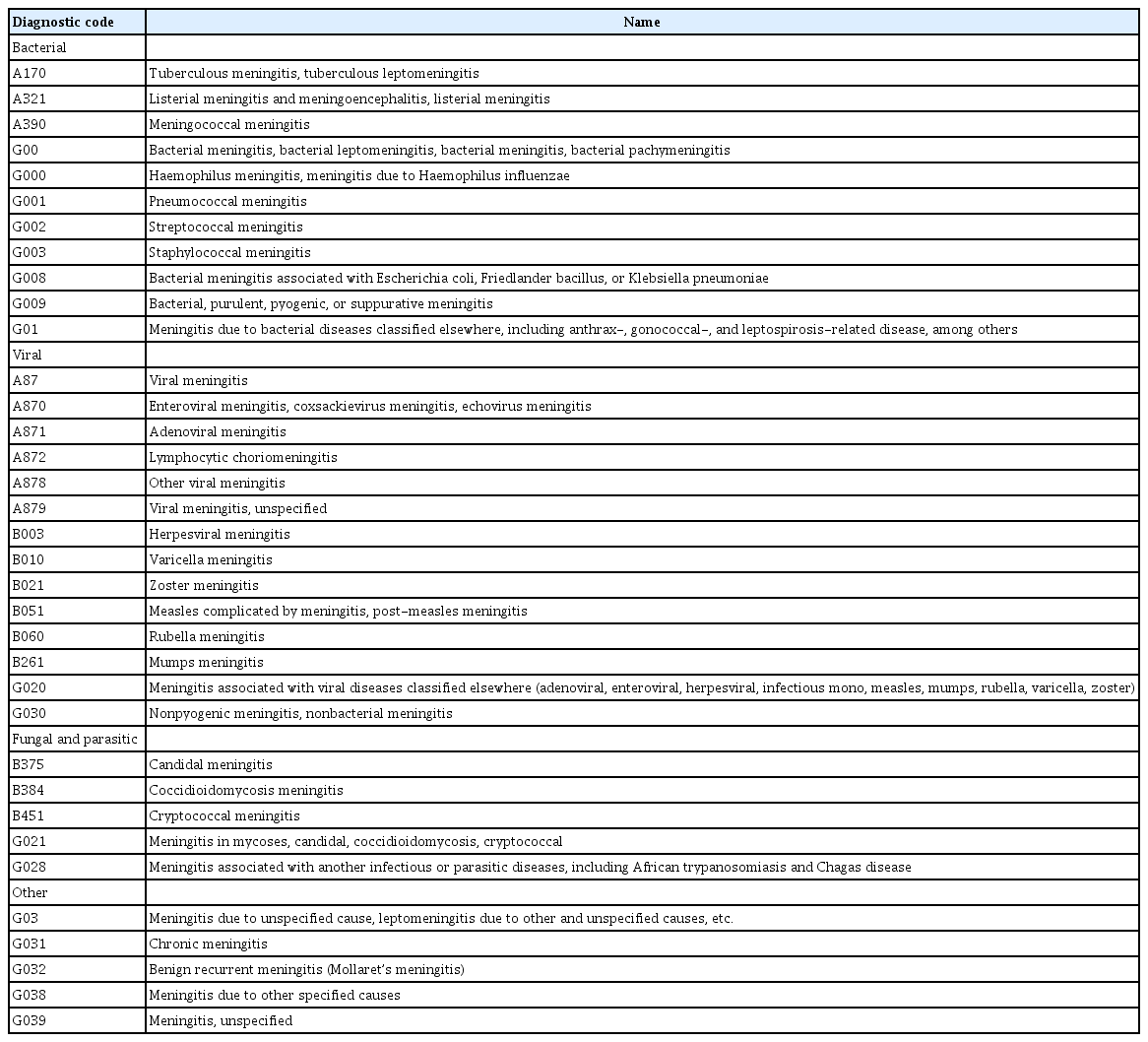
In this study, disease classification was performed according to the Korean Standard Classification of Disease and Cause of Death (KCD-7), which was revised in 2015, based on the World Health Organization's International Classification of Disease and Cause of Death [17]. We extracted the medical and demographic data of patients with meningitis who were registered with the HIRA from 2009 to 2017; this disease included 43 distinct entities. Diagnoses of meningitis were classified as bacterial, viral, fungal and parasitic, and other types; diagnoses with 0 patient were excluded (Table 1).
2. Study population
Domestic pediatric patients aged 0 to 18 years were included and classified based on their diagnoses (Table 1). In total, the data on 19,277 cases of bacterial, 155,959 cases of viral, 245 cases of fungal and parasitic, and 158,056 cases of other types of meningitis were extracted. Duplicate records were removed.
In general, meningitis is diagnosed and treated in an in-patient setting; thus, this study only included records from certified tertiary hospitals, general hospitals, and other types of hospitals. In addition to the above reasons, in this paper, since the HIRA database was used through the KCD-7 code, we tried to exclude patients who had diagnosed names only by clinical diagnosis. At this stage, the study sample comprised 2,166 cases of bacterial, 36,155 cases of viral, 30 cases of fungal and parasitic, and 46,192 cases of others type of meningitis (Fig. 1).
The same method was applied to extract and categorize each diagnosis into subgroups. The HIRA data only classified patients by their respective age, and not according to different age groups. However, in our analyses, we followed the staging system of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which classifies children based on age ranges [18].
3. Statistical analysis
Data were extracted from the HIRA database and examined using SAS Enterprise version 9.2 (SAS Institute, Cary, NC, USA). All statistical analyses were performed using SPSS version 20 for Windows (IBM Co., Armonk, NY, USA). Between-group differences were compared using t-tests, and P values <0.05 were considered significant.
Results
1. Monthly and annual meningitis frequency of patients
The monthly and annual number of pediatric meningitis in South Korea are reported in Supplementary Table 1. The monthly patient’s number peaked during the summer months (June, July, and August), with the highest number of cases registered in July. The total number of patients with meningitis and the major diagnosis of bacterial/viral meningitis correlated significantly during summer (P<0.001), especially in July (Supplementary Fig. 1).
2. Age-stratified number & frequency of bacterial and viral meningitis
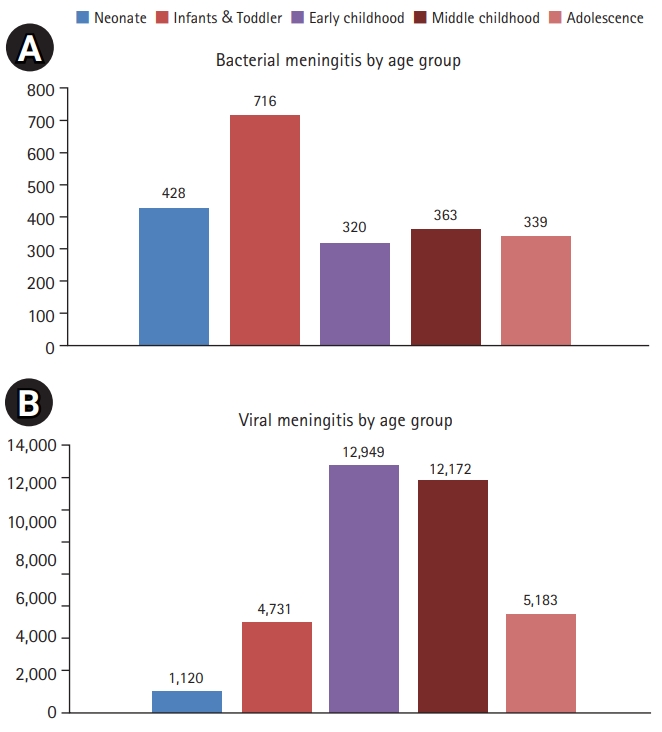
Bacterial meningitis was the most common type among infants; the number of cases in early childhood and that in adolescent age groups was similar (Fig. 2). When analyzing the number of patients against the total population, infants, neonates, childhood, and adolescence were in order. Viral meningitis was the most common in early/middle childhood (Fig. 3). Like bacterial meningitis, viral meningitis was in the same order when comparing the population by age group.
3. Most common disease types
Among bacterial meningitis types, the total numbers of children registered with the disease codes G009 (bacterial, purulent, pyogenic, or suppurative meningitis), G008 (bacterial meningitis associated with Escherichia coli, Friedlander bacillus, or Klebsiella pneumonia), and G002 (streptococcal meningitis) were 1,261 (58.2%), 375 (17.3%), and 263 (12.1%), respectively. In addition, there were 79 cases (3.6%) of A170 (tuberculous meningitis, tuberculous leptomeningitis) disease, which was the most common in adolescence. Finally, among infants, there were 245 cases of G002, which made it more common than G008 (141 cases).
Among the different types of viral meningitis, A879 (viral meningitis, unspecified) was the most commonly reported diagnostic code (16,574 [45.8%]), followed by A870 (enteroviral meningitis, coxsackievirus meningitis, echovirus meningitis; 9,216 [25.5%]), G030 (nonpyogenic meningitis, nonbacterial meningitis; 4,648 [12.9%]), and A878 (other viral meningitis; 3,693 [10.2%]). Although the specific viral species were recorded in a small number of cases, most cases involved adolescents with mumps (B261), zoster (B021), herpes (B003), and enterovirus infections. Among infants, herpesviral meningitis was the most commonly reported type (Supplementary Table 2). In case of fungal meningitis, the disease code G028 (meningitis associated with another infectious or parasitic diseases, including African trypanosomiasis and Chagas disease) was the most commonly reported, although it involved relatively few cases among infants and a comparable number of cases across the remaining age groups.
4. Sex-stratified disease frequency
Overall, there was a higher frequency of meningitis among boys (1%) than girls (0.70%), based on the ratio of male-to-female patients in the overall sample (51,573 vs. 32,970) and that in the general population of Korea (data for children aged 0 to 17 years in 2010: boy vs. girls, 5,135,542 vs. 4,711,252).
In a large category, classified as bacterial and viral meningitis, there was higher frequency of bacterial meningitis among boys (0.024%) than girls (0.020%) (1,247 vs. 919). Similarly with bacterial meningitis, there was higher frequency of bacterial meningitis among boys (0.43%) than girls (0.30%) (21,904 vs. 14,251).
Discussion
The present study investigated the data of bacterial and viral meningitis obtained from the Korean HIRA database to identify the temporal- and sex-related trends in the frequency of all-cause meningitis in children. In previous studies analyzing outbreaks from the late 90s to the early 2000s have shown that the most common meningitis-related pathogens were group B Streptococcus (GBS), Streptococcus pneumoniae, and H. influenzae [14,15]. Although the result is a single medical institution or several medical institutions, it can be considered a significant result.
A United States-based report on the etiology of bacterial meningitis during 1998 to2007 revealed that GBS, Listeria monocytogenes, and S. pneumoniae were the predominant pathogens causing meningitis among patients aged <2 months. Among children aged between 2 months and 2 years, S. pneumoniae, GBS, Neisseria meningitidis, and H. influenzae were the common cause of meningitis, while among children aged 2 to 17 years, S. pneumoniae, N. meningitidis, and H. influenzae were the most common causative agents [19,20].
A global meta-analysis that investigated meningitis etiology revealed S. pneumoniae as the most common cause of the disease among children. In Europe, meningitis due to N. meningitidis was the most prevalent among children aged 1 to 5 years, whereas that caused by E. coli and S. pneumoniae were the most prevalent among African neonates. Moreover, neonates in Europe had the highest prevalence of GBS infection, which was rare in the Eastern Mediterranean region [6]. Overall, the reported frequency differs between regions.
In the present study, the broadest diagnostic category corresponded to the disease code G009; several other diagnostic entities were included, and G008 included E. coli infections. Among cases in which the pathogen was identified, streptococcal meningitis was the most common among infants, which was consistent with domestic epidemiology.
Many H. influenzae outbreaks have been reported previously [14,15]; however, there have been relatively few outbreaks in the last 10 years. Of note, the code A170 appeared more than the other diagnostic names in adolescence, which may be due to the higher overall prevalence of tuberculosis in Korea [21].
However, pathogens that belong to the disease code G000 (Table 1), associated with H. influenzae (which used to be common), were rarely reported in the present study. This finding might be accounted for by the introduction of a national immunization support program for children (eight types) in 2009, with an additional anti-Hib vaccination administered since 2013 [22].
Immunization of children is effective for disease prevention worldwide. From 2000 to 2015 in particular, the frequency and mortality rates of meningitis due to S. pneumoniae and type B H. influenzae were reduced following the active implementation of conjugate vaccines worldwide [23,24]. In addition, in Japan, which shares climate and infectious disease characteristics with Korea, a recent study has shown that the frequency of bacterial meningitis has been decreasing since the last several years owing to increased vaccinations against H. influenzae and S. pneumoniae [25]. This finding is considered statistically significant and is consistent with trends observed elsewhere.
The enterovirus family is the leading cause of viral meningitis, followed by mumps and herpes simplex virus [10,26,27]. Mumps commonly occurs in adolescent children, whereas herpesviral and varicella-zoster virus meningitis tend to present among infants; these findings are consistent with those previously published in Korea [12,27,28].
The age-dependent frequency rate showed that viral meningitis was equally likely to occur across all age groups; however, bacterial meningitis was most likely to occur in infants [9,10,12,29,30]. Both types of meningitis were most likely to occur during the summer season, particularly in July. Viral meningitis was strongly associated with enterovirus infection, which is prevalent during summers. The frequency of bacterial meningitis followed a similar seasonal pattern [26,30-32]. This finding is likely due to the fact that bacteria such as E. coli or Klebsiella spp. (associated with the G008 diagnostic code), which account for a large proportion of bacterial meningitis cases, show an increased survival rate (by 3.5% to 8%) with a temperature increase by 5.6°C.
Owing to the large influence of temperature, infections during summer seemed to occur more vigorously than during winter [33,34]. The major strength of this study is its use of a large-scale database derived from health insurance claims, which allowed us to examine meningitis frequencies in detail. However, this study has some limitations. First, since the diagnosis recorded in the database is based on KCD-7 codes, diagnostic details relevant to meningitis (e.g., type of pathogen) were often unavailable. There is a disadvantage in using statistics to identify bacteria or viruses that cause a specific outbreak. For example, even if E. coli is detected, it is difficult to evaluate all the causes of the outbreak because the diagnosis code G008 (Other bacterial meningitis; due to E. coli, F. bacillus, Klebsiella) includes infection caused by all related bacteria, without detailed diagnosis.
Second, a diagnostic code such as G039 (meningitis, unspecified) corresponding to other diagnostic names may be clearly detected as meningitis in a medical institution, but unspecified diagnostic names may only reflect clinical symptoms. To maximally exclude these errors, only the data of patients who were admitted to the hospital were extracted; however, this error cannot be avoided.
Third, as our data source only included the data of the main diagnosis, cases involving meningitis that progressed to other diseases (e.g., sepsis) might have been missed.
In summary, this study examined meningitis frequency data using population-wide information derived from a medical insurance claims database. Further studies are required to elucidate the relationship between meningitis and other infectious diseases, including ones with analyses that address the current study limitations. Taken together, this evidence will contribute toward a better understanding of childhood meningitis in Korea.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.26815/acn.2020.00178.
Annual and monthly number of meningitis among children in Korea (2009 to 2017)
Meningitis incidence classified by month. VIR, viral; BAC, bacterial.
Number of meningitis types as per diagnostic codes among children in Korea during 2009 to 2017 [18]
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: SAC. Data curation: JWK. Formal analysis: SAC. Funding acquisition: JWK. Project administration: SAC. Visualization: SAC. Writing-original draft: JWK. Writing-review & editing: JWK, SAC, SYK, NML, DYY, SWY, and ISL.
Acknowledgements
We wish to thank Editage for English language editing assistance; we also thank the Institutional Review Board for their approval of this study.