FLNA Duplication in a Female Infant with Periventricular Nodular Heterotopia
Article information
Filamin A (FLNA) is involved in several fundamental processes surrounding cellular protrusion and motility [1]. Recent studies have shown that FLNA mutations can result in periventricular nodular heterotopia (PNH) [1], which is characterized by the failure of neurons to migrate appropriately during corticogenesis. This failure in migration causes nodular tissue formation in the ventricular lining of the brain [1]. It is known that FLNA mutation (Mendelian Inheritance in Man #3000049)-related PNH is usually inherited in an X-linked manner [2], such that hemizygous males run a high risk of intrauterine and perinatal mortality, whereas heterozygous variant-afflicted females present variously from asymptomatic to neurological symptoms (e.g., seizure and weakness) that preserve psychomotor function [3]. However, the mechanism of FLNA interaction with migrating neurons remain unknown [1].
Here, we report a case of an 8-month-old girl with FLNA-related PNH who was admitted for ocular deviation and unilateral motor weakness. She was previously healthy without a history of seizures and intellectual disabilities and had appropriately reached developmental milestones. She had received an influenza vaccination 1 week prior to admission and experienced a fever for 3 days after vaccination. A neurological examination showed reduced motor tone (grade 4) in the left arm and leg. Cerebrospinal fluid analysis and laboratory results were normal; however, magnetic resonance imaging (MRI) of the brain revealed PNH in the bilateral ventricles as well as acute disseminated encephalomyelitis (ADEM) (Fig. 1). She presented no abnormal range of motion and definite skeletal abnormalities such as shortened digits or hyperflexible joints. Her echocardiogram showed mild aortic valve regurgitation (trivial–grade 1). Ophthalmologic evaluation was within the normal range without symptoms such as strabismus. She was administered high-dose intravenous glucocorticoids therapy, and since it had little effect, was added intravenous immunoglobulin. In a follow-up brain MRI scan taken eight days later, corresponding improvements were observed with respect to acute neurological symptoms and ADEM lesions; however, the bilateral PNH remained. To evaluate the genetic cause of the cerebral lesions, we performed whole exome sequencing, but no significant single nucleotide variants were detected. So, copy number variation analysis were performed using the whole exome sequencing data. Heterozygous FLNA duplication (hg19 chrX: g.153,599,242–153,609,557) was identified and confirmed by quantitative real-time polymerase chain reaction validation. Unfortunately, this report has limitations in that parental genetic testing has not been performed.
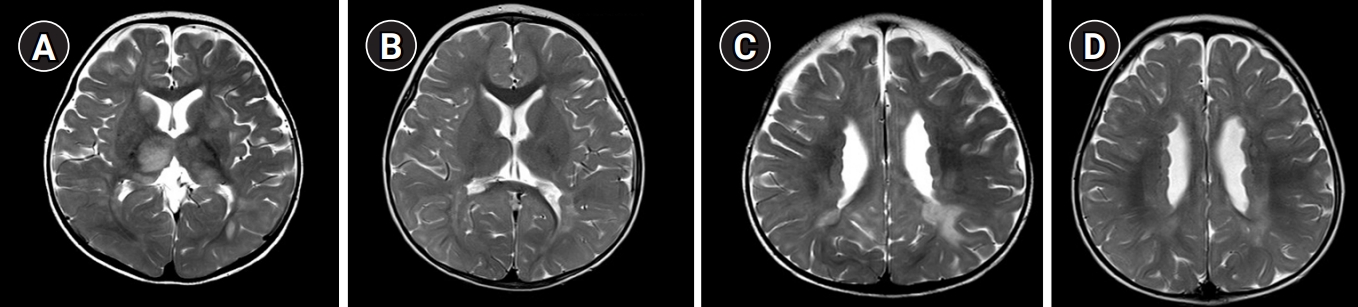
Brain magnetic resonance imaging scan in a female patient with filamin A (FLNA) gene duplication who had acute disseminated encephalomyelitis (ADEM) and periventricular nodular heterotopia. (A, C) T2-weighted images showing periventricular and subcortical white matter lesions in both the basal ganglia and thalami. (B, D) After 1 year, bilateral periventricular nodular heterotopia remained. the ADEM resolved.
Few cases in male and female PNH with FLNA deletion have recently been found. However, although there has been one reported case of PNH in a patient with a large duplication copy number variant that included the FLNA gene [3], this is the first reported case of a female patient with PNH who had duplications in exons 1 and 2 of the FLNA gene. Thus, this study expands the genotype–phenotype spectrum for FLNA variants associated with PNH. It also demonstrates the importance of using diagnostic tools for detecting copy number gain as well as deletion and sequencing variants in the FLNA gene.
Informed consent was waived by the board and this study was approved by the Institutional Review Board of Yonsei University Health System (IRB, 3-2020-0373).
Notes
Young-Mock Lee is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contribution
Conceptualization: HL and YML. Data curation: NSK, HL, and YML. Visualization: HL. Writing-original draft: NSK and HL. Writing-review & editing: HL and YML.
