Nusinersen Administration in Spinal Muscular Atrophy Patients with Severe Scoliosis: Interlaminar Approaches at the Lumbar Level
Article information
Abstract
Purpose
Spinal muscular atrophy (SMA) is a genetic progressive neuromuscular disorder, and nusinersen has shown to improve its symptoms. Scoliosis is a frequent symptom in patients with SMA and complicates the intrathecal injection of nusinersen. The aim of this study was to investigate the safety and effectiveness of fluoroscopy-guided intrathecal injections in patients with SMA with severe scoliosis.
Methods
We retrospectively reviewed the medical records of 11 patients with SMA treated with nusinersen intrathecal injections at the Samsung Medical Center from 2018 to 2020. We assessed the type of SMA, genetic results, spine computed tomography or fluoroscopy findings, and adverse effects associated with the injection.
Results
Of 11 patients with SMA, six were diagnosed with severe scoliosis, four of whom underwent an operation for scoliosis. The six patients with severe scoliosis started receiving the nusinersen injection at a median age of 15.7 years. A total of 25 injections were completely performed with the interlaminar approach (ILA) at the lumbar level under C-arm fluoroscopy guidance. No adverse effects other than mild headache occurred. In one patient who underwent the complete fusion operation for scoliosis, laminectomy was performed for the nusinersen injection, and 3 doses were administered intrathecally via the interlaminar route at the lumbar level.
Conclusion
Fluoroscopy-guided ILA is a safe method for the intrathecal injection of nusinersen in patients with SMA with severe scoliosis. When defining the route, laminectomy might be necessary to open the window for the ILA route at the lumbar level.
Introduction
Spinal muscular atrophy (SMA) is a hereditary progressive neuromuscular disorder. Most patients with SMA present with homozygous disruption of the survival motor neuron 1 (SMN1) gene on chromosome 5q13.2 [1]. The SMN protein is encoded identically by both SMN1 gene and SMN2 gene. However, SMN2 gene only produces 10% to 15% of all functional SMN proteins with typically alternating splicing [2,3]. Insufficient SMN protein levels cause progressive spinal anterior horn cell degeneration. Nusinersen (Spinraza, Biogen, Cambridge, MA, USA) modifies the pre-messenger RNA splicing of SMN2 to prevent the exclusion of exon 7 and increases the expression of the SMN protein [4]. However, nusinersen is an oligonucleotide that is limited to penetrating the cell membrane and needs to be administered locally through an intrathecal injection [5].
Scoliosis occurs commonly in neuromuscular disorders and decreases the vital capacity of the lungs by worsening the expansion of the chest wall. Patients with SMA frequently present with scoliosis as the general course of disease. Scoliosis develops in all types of non-ambulant patients with SMA from early childhood. Scoliosis is usually considered severe when the spinal curve measured with the Cobb method exceeds 40° to 50°. Progressed scoliosis requires surgery, mostly posterior corrective spinal fusion surgery in adolescence [6,7].
Nusinersen is an expensive drug and required a secure and complete administration route. The posterior interlaminar approach (ILA) is the representative and safe method for the intrathecal administration of the drug. Patients with scoliosis or spinal deformity require image-guided technique for this approach. Certain methods of spinal fusion surgery make it difficult to approach because it causes the obstruction of the interlaminar space even under imaging guidance. Various methods for the intrathecal approach have been developed, but each method has reported adverse effects. Therefore, evaluation of the security for the nusinersen injection requires increased follow-up time and evidence. The objectives of this study were to investigate the safety and effectiveness of fluoroscopy-guided nusinersen intrathecal injections for SMA patients with severe scoliosis, including the safety and effectiveness of laminectomy for creating the route for ILA necessitated by obstruction of the interlaminar space after complete fusion operation of the whole spine.
Materials and Methods
We enrolled patients diagnosed with SMA and scheduled for a nusinersen injection in the period from 2018 to 2020 at the Samsung Medical Center. Inclusion criteria were same with the Korea National Health Insurance Service indication for nusinersen as follows: the presence of the clinical manifestation before the age of 3 years; and the presence of the SMN2 copy. Exclusion criteria were based on the usual respiratory function. The patients, who necessitate the artificial ventilation for consecutive 21 days and 16 hours per day, were excluded. The use of artificial ventilator during the acute infection including pneumonia, was not counted for the evaluation.
We retrospectively reviewed their medical records, including the age of onset of the first symptom, spine computed tomography (CT) or fluoroscopy findings, results of genetic testing, including copy numbers of SMN1 and SMN2 genes, and motor function at each nusinersen therapy. We performed the Multiplex ligation-dependent probe amplification for finding the copy number variation of SMN1 and SMN2 genes.
Nusinersen injections were started with four times of loading injection. The first three doses were given by 2-week intervals and the fourth dose was administered 5 weeks after the 3rd injection (on week 0, 2, 4, 9). Maintenance doses have been given by 4-month intervals. Regardless of the patient’s body weight or body surface area, 12 mg (5 mL) of nusinersen was administered in each according to the manufacturer’s guide. All patients applied local anesthetic ointment (lidocaine and prilocaine) on the skin corresponding to L3–4 and L4–5 level at least 30 minutes before the procedure. Of 11 patients, four needed the sedative medication for poor cooperation or the fear of the procedure and they were given one dose of intravenous midazolam (0.05 mg/kg, maximum 3 mg) just before the procedure under the continuous electrocardiography and oxygen saturation monitoring until that patient was fully awakened and maintained stable respiration. After confirming the spinal needle located in the cerebrospinal fluid (CSF) space, we drained 5 mL of CSF according to the manufacture’s guide, which is the same amount as the injection dose of nusinersen, and administered the medication slowly over 2 minutes. After the procedure, the patients were encouraged to lie in the supine position and provided the physical pressure to injection site with gauze or pillow for more than 2 hours.
For SMA patients with severe scoliosis, C-arm fluoroscopy (Siemens Artis Zee System, Erlangen, Germany) was performed for nusinersen infusion. Each patient was placed in the lateral decubitus or supine position according to the curvature seen on the spine CT. C-arm fluoroscopy visualized the location of spinal needle and each insertion angle was decided depend on each image of fluoroscopy.
We analyzed the response to treatment based on the Hammersmith Infant Neurological Examination section 2 [8] for patients under the age of 24 months or development performance corresponding to the age of 24 months and Hammersmith Functional Motor Scale Expanded [9] for patients above the age of 24 months. We assessed scoliosis by measuring the spinal curve with the Cobb method. Severe scoliosis was defined as a spinal curve angle over 30º which affects the posture on physical examination and is expected to pose difficulty in the ILA at the lumbar level. The respiratory function was measured indirectly by venous blood gas analysis. First, we checked the vital signs and chest X-ray findings during the admission period. We additionally performed venous blood gas analysis for the patients being supported with artificial ventilator or presenting the respiratory difficulty including chest retraction or tachypnea. Conventional spirometry was performed in only one patient (Patient 11) for evaluation of the initial respiratory function and showed the severe restrictive pattern. Other patients did not perform the additional pulmonary function test.
This study protocol was approved by the Institutional Review Board of the Samsung Seoul Hospital (IRB file No. 2020-02-115). Informed consent was waived by the board.
Results
Eleven patients with SMA were included in this study: one with type 1 SMA and 10 with type 2. Nusinersen administration was started at a median age of 10.1 years (range, 0.4 to 22.6; mean, 10.3). Five patients had no or mild scoliosis because they were less than 7 years of age at the start of this study. The other six patients had severe scoliosis, four of whom had undergone a scoliosis surgery. The number of nusinersen injections per patient was the median five times (range, 4 to 9) in all 11 SMA patients and the number of fluoroscopy-guided injections per patient was the median of five times (range, 1 to 5).
Six patients with scoliosis started receiving the nusinersen injection at a median age of 15.7 years (range, 10.1 to 22.6; mean, 15.7) (Table 1). A total of 25 intrathecal injections were administered under C-arm fluoroscopy guidance without general anesthesia or respiratory support. To prove the accessibility to the CSF space insuring the perfect intrathecal injection of nusinersen, we performed lumbar spine CT for five patients, which confirmed the accessibility to the CSF space in all patients (Fig. 1). One patient showed no accessible route because of complete fusion of the posterior portion of the lumbar spine.
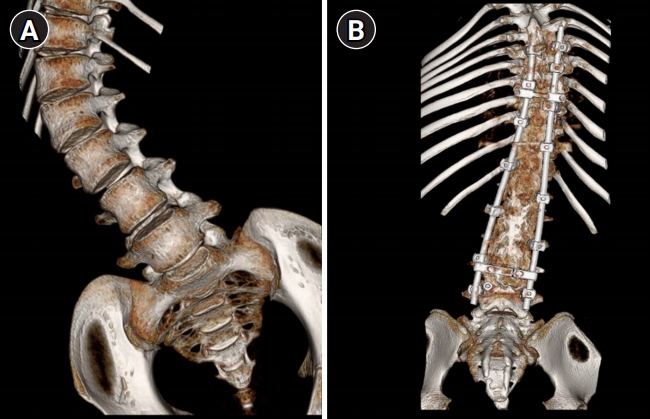
Three-dimensional reconstruction of spine computed tomography. (A) Patient 5 with spinal muscular atrophy (SMA) presented a scoliosis and no history of operation for scoliosis. (B) Patient 9 with SMA presented a scoliosis and history of posterior instrumentation with partial fusion at the central canal.
Four patients underwent the fusion operation for scoliosis at a median age of 10.3 years (range, 10 to 10.5; mean, 10.3) and started receiving the nusinersen injection at a median age of 19.2 years (range, 13.5 to 22.6; mean, 18.60) (Table 2). For them, a trial injection was administered under C-arm fluoroscopy guidance, which can offer cone beam CT acquisition when necessary, by checking for a direct access to the CSF space. Three patients (Patients 8, 9, and 10) were operated with posterior instrumentation, two of whom (Patients 8 and 10) showed partial fusion of the central canal and facet joint with preserved interlaminar space at the lumbar level. Approaching the CSF space was successful through the interlaminar path. A total of 12 intrathecal injections were administered under C-arm fluoroscopy guidance (Fig. 2).

Fluoroscopy of spinal muscular atrophy patients with severe scoliosis. All fluoroscopy-guided procedures are performed with the interlaminar approach at the lumbar level. (A) Patient 5 presents with severe scoliosis not treated with an operation and is administered with an intrathecal injection at the age of 10.1 years. (B) Patient 9 had undergone posterior instrumentation with partial fusion for scoliosis at age of 10.5 years and has started receiving intrathecal injections at the age of 20.8 years.
The approach was impossible in one patient (Patient 11) because of complete fusion of all the posterior elements of the thoracolumbar spines. She presented with delayed motor development and motor weakness at the age of 18 months and was diagnosed with type II SMA. At the age of 10 years, her scoliosis was severe (Cobb angle, 92.6°). At the age of 10.5 years, she underwent scoliosis operation (Fig. 3). Because of the complete fusion operation for scoliosis, typical ILA at the lumbar level was impossible, and coccygeal approach was also impossible for assessing the intrathecal cavity. Laminectomy was performed at the L2 and L3 levels to secure the route for nusinersen injections. Three days after laminectomy, we confirmed an assessable route at the L3 level under C-arm fluoroscopy guidance (Fig. 3). Three doses of nusinersen therapy were successfully administered without any adverse effect, such as stenosis or leakage from the intrathecal route.

Fluoroscopy after laminectomy: Patient 11. Patient 11 had undergone posterior instrumentation with complete fusion for scoliosis at the age of 10.5 years and laminectomy at the age of 17.4 years. (A) Figure shows a lumbar spine computed tomography scan in the sagittal view performed at the age of 17 years. (B) Figure shows an intraoperative image during laminectomy performed at the age of 17.4 years. (C) Figure shows a cone-beam computed tomography scan acquired during fluoroscopy performed 3 days after laminectomy. A spinal needle is approached into the interlaminar space at the L3 level. (D) A three-dimensional reconstruction image is acquired during fluoroscopy for confirming the possibility of the intrathecal approach.
1. Intrathecal injection-related adverse effect
No serious adverse effects related to intrathecal injections of nusinersen occurred. Two patients had headache for 2 days after the injection, which was controlled with hydration and non-steroidal anti-inflammatory drugs.
Patient 5 with type II SMA and severe scoliosis (Cobb angle, 53.1°) had headache and dizziness for 2 days after the intrathecal injection. Decreased oral intake of food after the injection might induce headache, and sufficient hydration could prevent the occurrence of headache in following injections.
Patient 8 with type II SMA and scoliosis operation had headache for 2 days after the intrathecal injection, which was controlled with acetaminophen. The intensity of headache was mild and did not affect the performance of activities of daily living.
Discussion
This study showed successful intrathecal injections of nusinersen under C-arm fluoroscopy guidance in six SMA patients with severe scoliosis. Nusinersen injections were administered successfully in four patients who had undergone the posterior fusion operation for scoliosis. In one patient who had undergone the complete fusion operation, the approach to the CSF space was obstructed even under fluoroscopy guidance. However, the CSF space was successfully accessed after laminectomy. No procedure-associated serious adverse effects other than mild headache occurred.
The spinal anatomy influences the intrathecal injection of nusinersen. As SMA progresses, spinal deformity worsens; therefore, anatomical distortion becomes a hurdle for the intrathecal approach and required real-time imaging guidance for approaching the interlaminar route [10-14]. Furthermore, multiple operations of spondylodesis might cause ossification of the puncture site, necessitating the consideration of approaches other than a direct puncture [11].
Ultrasound and fluoroscopy can be utilized for real-time imaging-guided approaches. Ultrasound has a limitation of view due to artifacts associated with instrumentation used in the scoliosis operation and thick cutaneous or subcutaneous tissue around the approaching site [15]. Fluoroscopy provides superior images compared to ultrasound but still has the potential risks associated with accumulated radiation [16]. We successfully administered 25 times of intrathecal injections in six SMA patients with severe scoliosis via the interlaminar route under fluoroscopy guidance for up to 8 months. None of the patients developed stenosis or ossification associated with the intrathecal injection.
As for the anatomic approach route, ILA, transforaminal approach (TFA), and cervical level approaches have been reported [12-14]. The transforaminal space is commonly used for epidural steroid injections and is the only route directly assessable to the intrathecal space in a patient with fusion of interlaminar route. Up to 42 TFA cases were successfully completed without complications, and 30 cervical punctures were performed without bleeding or infection with the cervical puncture (Table 3). However, one patient reportedly developed subarachnoid hemorrhage associated with TFA at the lumbar level. TFA might increase the risk of nerve or vascular injury, which causes more severe neurologic complications, especially at the cervical level, including quadriparesis, stroke, and death [17]. In addition, implantation of an intrathecal port system can be considered in SMA patient with profound scoliosis for reducing sedation and radiation exposure induced by fluorocopy [18]. However, this required follow-up to evaluate probable risks, such as of infection or obstruction. To date, ILA has been the dominant route being safe and efficient for nusinersen injections [10]. Many reports have suggested the safe and definite intrathecal route for scoliosis patients with type 2 or 3 SMA, and our study demonstrates the ILA injection. All our patients underwent injections with ILA at the lumber level. Our study included a relatively young patient diagnosed with type 2 SMA and severe scoliosis. Unlike older patients, younger ones need the safest method for these approaches, considering compliance during the procedure. ILA at the lumbar level might be considerd as the most appropriate approach, and reconstruction for that route is preferred to finding another approaching route.
Surgical treatments for scoliosis are necessary for the improvement of posture and lung capacity. Conventionally, the posterior fusion operation has been the most popular. However, a fusion mass can obstruct the lumbar ILA, and two related studies reported the operation to open the bone window for ILA at the lumbar level [19,20]. In our study, one patient (Patient 11) was treated with posterior instrumentation and complete fusion for scoliosis, which hindered the window for ILA at the lumbar level. We decided to open the bone window with laminectomy at the L2–L3 level, rather than TFA or cervical puncture. This patient was confirmed the interlaminar route by fluoroscopy, 3 days after laminectomy, and, three loading doses were completely administered with fluoroscopy-guided ILA without adverse outcomes, such as post-operative or post-injection CSF leak or pseudo-meningocele. The laminectomy window enabled a safe lumbar puncture but still has a potential to weaken the support with the remaining hardware or rod used for mechanical stability. This approach requires close monitoring for stability. Simultaneously, other supplements, such as fat transplantation or artificial dura, may be necessary to secure the bone window without obstructing the route [21].
In this study, we did not assess the improvement of motor function or cost-effectiveness of nusinersen because of short follow-up duration. There are various opinions on the treatment with nusinersen in SMA patients with low level of motor function as disease progresses. Nusinersen is not curative medication and theoretically requires lifelong intrathecal injection. In patients showing the significant improvement to treatment, maintaining the injection of nusinersen is reasonable, however, lofty price is an economic burden. On the other hand, restricting nusinersen treatment to patients, who are in the advanced stage of disease or show the minimal response, could be an ethical problem [22]. Even with evidences about the safety and effectiveness proved by clinical trials, repetitive invasive injections and cost-effectiveness would require further investigations.
This study illustrated successful fluoroscopy-guided intrathecal injections of nusinersen with ILA in a relatively large number of patients with severe scoliosis. There were no short-term adverse effects associated with fluoroscopy, laminectomy for the bone window, or ILA at the lumbar level. ILA at the lumbar level is the safest method for intrathecal injections, and fluoroscopy guidance was necessary for an accurate approach in patients with severe scoliosis. In addition, laminectomy for creating the window of ILA can also be considered based on the previous operation method. For further evidences, longer follow-ups and a larger number of patients are necessary for the evaluation of stability of the post-laminectomy state and safety of repeated fluoroscopy-guided lumbar punctures. In conclusion, fluoroscopy-guided ILA is safe and effective for the intrathecal administration of nusinersen in SMA patients with severe scoliosis. Future studies with a larger number of patients are warranted.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: JYS, JL, and JL. Data curation: JYS. Formal analysis: JYS, JL, and JL. Methodology: JYS, HSK, SJP, and JL. Project administration: JYS and JL. Visualization: JYS. Writing-original draft: JYS, JL, and JL. Writing-review & editing: HSK, SJP, JL, and JL.
Acknowledgements
We thank the patients and their families for participation and cooperation during the study. No funding was received for this study.



