Clinical Spectrum of Posterior Reversible Encephalopathy Syndrome in Children
Article information
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a complex neurological condition characterized by the acute onset of neurological symptoms, such as seizures and potentially reversible vasogenic edema with preferential involvement of the parieto-occipital region in brain magnetic resonance imaging. Although PRES can present with a wide spectrum of clinical manifestations, the most common are seizures, visual disturbances, headaches, and altered mental states. PRES has been recognized in a wide variety of clinical settings including hypertension, immunosuppressants following organ transplantation, chemotherapy, renal diseases, sepsis, and autoimmune disorders. It has been increasingly reported even in children, but many aspects of this syndrome are incompletely understood and validated diagnostic criteria is still lacking. In this review, we will cover putative pathophysiological mechanisms, etiologic categories, clinico-radiological manifestations, and outcomes from previous studies.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiographic syndrome with different etiologies that are grouped together and referred to as posterior leukoencephalopathy syndrome, reversible posterior leukoencephalopathy syndrome, brain capillary leak syndrome, or hyperperfusion encephalopathy. PRES was first recognized in 1996 in patients who had renal insufficiency or hypertension or who were immunosuppressed [1]. PRES is characterized by a sudden onset of neurological symptoms, such as seizures, headache, visual disturbances, and reversible, cortical/subcortical vasogenic edema, commonly involving the parieto-occipital region of the brain [2-4]. Although the pathophysiology remains controversial, acute endothelial damage and subsequent interstitial brain edema is thought to cause PRES in patients with comorbid conditions such as hypertension crisis, kidney disease, use of cytotoxic drugs, immune mediated diseases [5,6]. Generally speaking, PRES has a good prognosis and is often reversible if treated early and adequately.
PRES in children has been more frequently reported, but most of the reports were conducted as single-center, retrospective studies, with focused populations, and a limited number of patients. The estimated incidence of PRES in children ranges from 0.04% to 0.7% [7-9]. As expected, many aspects of PRES are incompletely understood in children. This review will discuss putative pathophysiological mechanisms, etiologies, clinico-radiological manifestations, and outcomes from a pediatric perspective.
Pathophysiological theories
Several theories have been proposed regarding the pathophysiological of PRES; these are summarized in Table 1. The vasogenic theory proposes that sudden, severe hypertension (i.e., exceeding the capacity of cerebral blood flow autoregulation) leads to endothelial injury, breakdown of the blood-brain barrier (BBB), and subsequent vasogenic edema [2,10]. On the other hand, vasospasm and resultant cerebral ischemia may develop neurological symptoms [6,11]. The cytotoxic theory proposes that the endothelial dysfunction of PRES is due to exogenic toxins, such as drugs for chemotherapy or immunosuppression, or endogenic toxins, such as those generated during sepsis or eclampsia [2,6,12,13]. The immunogenic theory proposes that endotheliopathy, mediated by T-cell activation and the release of cytokines such as tumor necrosis factor-α (TNF) and interleukin-1 (IL-1), increases endothelial permeability and subsequent vasogenic edema [3,4,10,14]. In addition, TNF-α and IL-1 induce astrocytes to produce vascular endothelial growth factor, which disintegrates tight junction and increases vascular permeability. Finally, the neuropeptide theory proposes that potent vasoconstrictive neuropeptides, such as endothelin 1 and thromboxane A2, induce severe vasoconstriction, endothelial dysfunction, and subsequent cerebral hypoperfusion, which can proceed to cerebral ischemia and edema [14]. However, evidence to support each of these theories is lacking. We therefore postulate that PRES can result from combinations of these mechanisms, at least in the initial phase.
Etiology/predisposing factors
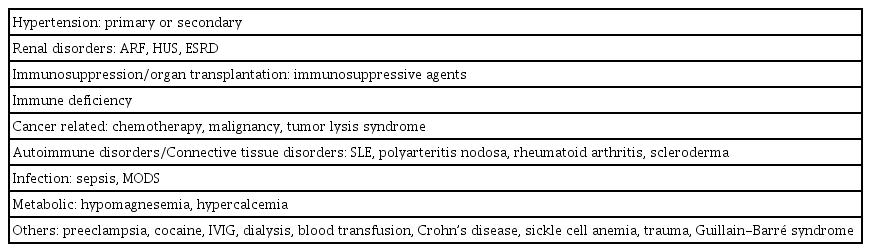
Initially, PRES was associated with immunosuppressive therapy, such as cyclosporine A after transplantation, or conditions in which blood pressure (BP) rises acutely [1,15-19]. Other comorbid conditions or risk factors of PRES have been reported and are becoming more frequent in children; these are summarized in Table 2. As in adult cases, primary or secondary hypertension and renal disease are the most significant predisposing factors in children and adolescents [6,9]. In addition, the use of immunosuppressive agents after organ transplantation or bone marrow transplantation, including tacrolimus, cyclosporine A, mycophenolate, and corticosteroids, has been a major risk factor [4,6,15,19,20]. PRES has also been reported in children with cancers, such as acute lymphoblastic leukemia or lymphoreticular malignancy, and/or the use of chemotherapeutic drugs such as vincristine, cyclophosphamide, cytarabine (Ara-C), cisplatin, methotrexate, and L-asparaginase [6,9]. It is not surprising that autoimmune disorders are one of the most common comorbidities [4,6,9,21]. Immunoglobulin or therapeutic monoclonal antibodies, such as rituximab, can be causative agents [6,22,23]. Furthermore previous studies in patients, including children, reported a significant association between blood transfusion and PRES [9,24,25]. Other predisposing factors include sepsis, hypercalcemia, hypomagnesemia, Guillain-Barré syndrome, Crohn’s disease, dialysis, and trauma [4,6,9,26].
Clinico-radiological features and diagnosis
Children with PRES typically present with sudden onset of neurological symptoms, such as seizures, visual disturbances, headaches, and altered mental states [1-3,19,27,28]. Focal or generalized seizures are the most common symptom, accounting for approximately 90% of children with PRES [4,29]. As with adults, headache is another common symptom, followed by visual disturbances, including hemianopia, blurred vision, cortical blindness, or visual hallucinations), altered mental state, hemiparesis, dysarthria/aphasia, ataxia, involuntary movements, dizziness/vertigo, and other various focal neurologic signs [4,19,27,29].
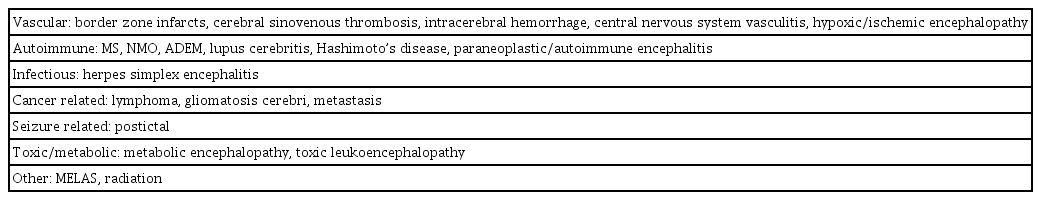
The typical magnetic resonance imaging (MRI) findings of PRES can be recognized as cortical and subcortical, fairly symmetric, T2 or fluid-attenuated inversion recovery hyperintensities, primarily in the parieto-occipital region. These features can be caused by vasogenic edema, although some cases show additional involvement of frontal or temporal lobes (Fig. 1). Based on the distribution of vasogenic edema, four major patterns have been described. These patterns are the classic parieto-occipital pattern in the middle cerebral artery (MCA) and posterior cerebra artery border zone, the superior frontal sulcus pattern predominantly along the anterior cerebral artery-MCA watershed zone, the holohemispheric watershed pattern, and the central pattern located predominantly in the deep white matter, basal ganglia, thalami, and brain stem in a minority of children with PRES [4,30]. Fig. 2 shows an atypical case of PRES with multiple hyperintense lesions in the brain stem, especially the pons. Most such lesions resolve within days or weeks when the underlying causes are eliminated or treated appropriately. However, intracranial hemorrhage, cerebral infarction, hydrocephalus, and other irreversible injuries that lead to more severe neurological sequelae can be seen in some cases. Furthermore, it is not uncommon for MRI findings to not correlate with clinical severity. Diffusion-weighted imaging (DWI) with calculated apparent diffusion coefficient (ADC) maps help to distinguish vasogenic edema from cytotoxic edema or acute infarction. In general, PRES lesions are not well-defined by DWI, but are hyperintense in ADC maps (Table 3). With cytotoxic edema with diffusion restriction, very subtle or even normal MRI findings are seen in some cases. PRES and reversible cerebral vasoconstriction syndrome (RCVS) often coexist; therefore, it appears that PRES overlap with RCVS can be a complication of RCVS [31].
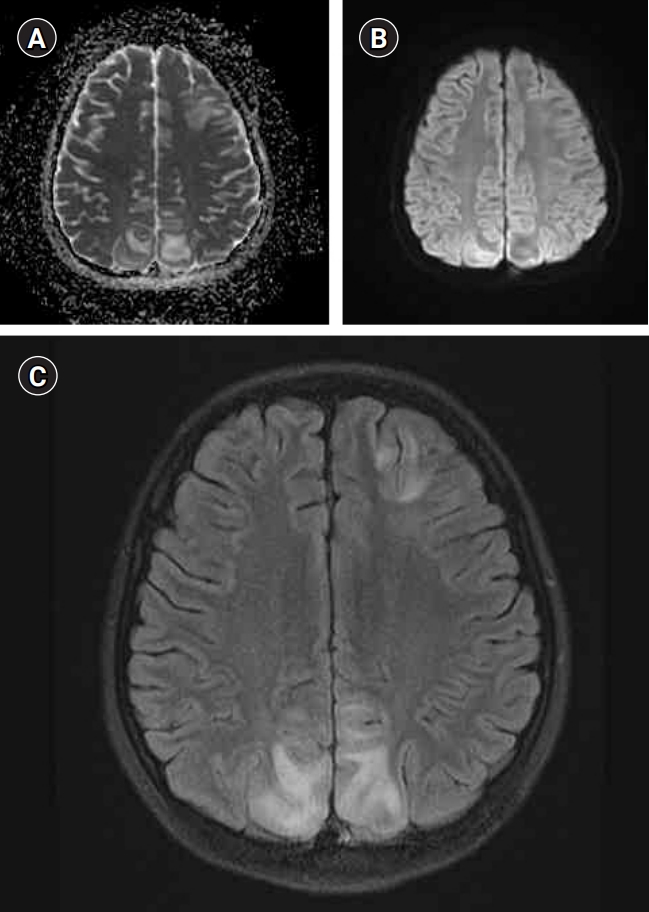
A 9-year-old girl with systemic lupus erythematosus presented with seizures. The magnetic resonance imaging shows typical findings of vasogenic edema in posterior reversible encephalopathy syndrome. (A) The apparent diffusion coefficient map shows multiple, hyperintense lesions, predominantly in biparietal regions. (B) The lesions are not well-defined in diffusion-weighted imaging. (C) Axial T2 fluid-attenuated inversion recovery sequences reveal multiple hyperintense lesions in the biparietal and left frontal regions.
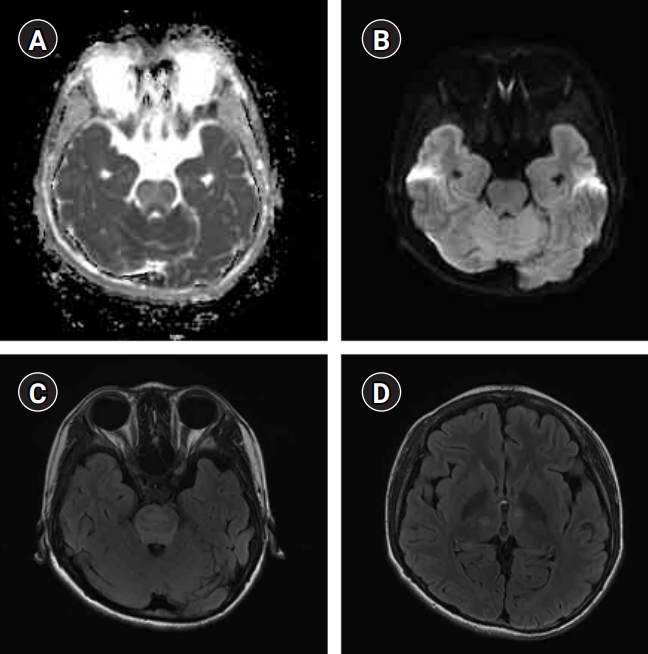
A 13-year-old boy with chronic kidney disease presented with headache and diplopia. The magnetic resonance imaging shows atypical findings of posterior reversible encephalopathy syndrome. (A) The apparent diffusion coefficient map shows multiple, hyperintense lesions in the brain stem, especially the pons. (B) The pontine lesion is not well-defined in diffusion-weighted imaging. (C, D) Axial T2 fluid-attenuated inversion recovery sequences reveal multiple, hyperintense lesions in the brain stem and bilateral thalami.
To date, validated guidelines and diagnostic criteria for PRES are still lacking; therefore, diagnoses are made by clinical judgement. Given the uncertainty of this judgement, brain MRI is crucial for diagnosing PRES and excluding similar neurological conditions. Differential diagnosis strategies for PRES in children and adolescents are shown in Table 4. Recently, a reasonable diagnostic algorithm for PRES has been proposed. Such an algorithm will help to identify atypical cases, and might increase our understanding of the full range of PRES [3]. The overall clinical and radiological features of PRES in children are similar to those in adults, with a few minor differences; therefore, the proposed diagnostic algorithm can be applied in children.
Recent studies have reported that albumin-cytologic dissociation of cerebrospinal fluid (CSF) occurs in most patients with PRES, and there is a positive correlation between the severity of vasogenic edema and the CSF protein level [32,33]. In addition, nonconvulsive seizures and epileptiform patterns, such as periodic localized or lateralized epileptiform discharges, predominantly over posterior regions are observed with continuous electroencephalography monitoring in 23 out of 37 (62%) patients with PRES [34].
Treatment
Robust clinical studies that identify effective therapies are lacking. As a result, PRES lacks evidence-based treatment regimens. However, in most cases, this syndrome typically resolves within days or weeks when the underlying causes are eliminated or treated appropriately. The most important first step is the prompt removal of underlying causes, such as the discontinuation of immunosuppressive agents when PRES is diagnosed or suspected. There is a general consensus that treatment of hypertension is important. The initial goal is to reduce BP by 20% to 30% with the first few hours. Patients should be continuously monitored in an intensive care unit to avoid pronounced fluctuations of BP. Acute seizures should be treated with intravenous antiepileptic drugs (AED), such as lorazepam, fosphenytoin, or levetiracetam, although no specific guidelines for AED treatment are available.
Prognosis and outcome
Generally speaking, PRES is a reversible neurological condition with a good prognosis if treated early and appropriately. However, despite the name, some clinical and radiological features are not fully reversible. Previous studies report complete recoveries only in approximately 70% to 90% of cases [35-37]. Most patients recover within a week, typically with hours to a few days, although some patients take several weeks to recover [37]. Atypical MRI findings such as hemorrhage and diffusion restriction are associated with poor outcomes [38].
Permanent neurological sequelae are reported in approximately 10% to 20% of patients and death can occur in 3% to 17% of cases [21,36,39]. Poor outcomes can result from intracranial hemorrhage, brain stem compression from posterior fossa edema, acute hydrocephalus, or severe whole brain edema. Recurrence occurs in approximately 5% to 10% of patients [40].
Conclusion
PRES is a complex clinico-radiological syndrome characterized by acute neurological symptoms, such as seizures and potentially reversible parieto-occipital vasogenic edema in brain MRI. PRES is caused by cerebral endotheliopathy with subsequent disruption of the BBB and vasogenic edema. PRES occurs in specific settings such as abrupt hypertension, renal disorders, the use of immunosuppressive drugs, or following organ transplantation. Brain MRI is crucial for diagnosing PRES, but validated diagnostic criteria have yet to be established. If the underlying cause is removed in the early phase, PRES resolves in days to weeks. However, neurological sequelae and even mortality can occur, particularly in patients with intracranial hemorrhages.
Although our knowledge of and experience in treating PRES have increased dramatically, many aspects remain unclear. Further studies are needed to elucidate the pathophysiologic mechanisms; such understanding could provide a backbone for clinical research and identify treatments that can cure PRES. From a clinical perspective, validated diagnostic tools or algorithms and possible treatment options should be developed from large, multicenter, prospective studies to improve clinical outcomes.
Notes
No potential conflict of interest relevant to this article was reported.
Authors’ contribution
Conceptualization: SKH and SK. Data curation: SKH, YJL, SML, and SK. Formal analysis: SKH, YJL, SML, and SK. Methodology: SKH, SML, and SK. Project administration: SKH, YJL, and SK. Visualization: SML and SK. Writing-original draft: SKH and SK. Writing-review & editing: SKH, YJL, SML, and SK.