|
 |
- Search
| Ann Child Neurol > Volume 28(1); 2020 > Article |
|
Guillain-Barr├® syndrome (GBS) is an acute inflammatory demyelinating polyneuropathy, usually presenting with proximal muscle weakness of the lower extremities and sensory dysesthesia, commonly triggered by preceding viral or bacterial infections. Cranial nerve involvement has been reported in 45% to 75% of patients with GBS, facial nerve involvement being the most common [1]. Facial palsy in GBS is often bilateral. Unilateral facial palsy is rare, and very few cases have been reported [2,3]. There are a limited number of pediatric GBS cases with unilateral facial palsy described in the literature [4]. To date, there has been one pediatric case report with unilateral facial palsy as an isolated and presenting feature of GBS [5]. Here we report the case of an infant with GBS, whose first presentation was unilateral peripheral facial palsy following gastroenteritis.
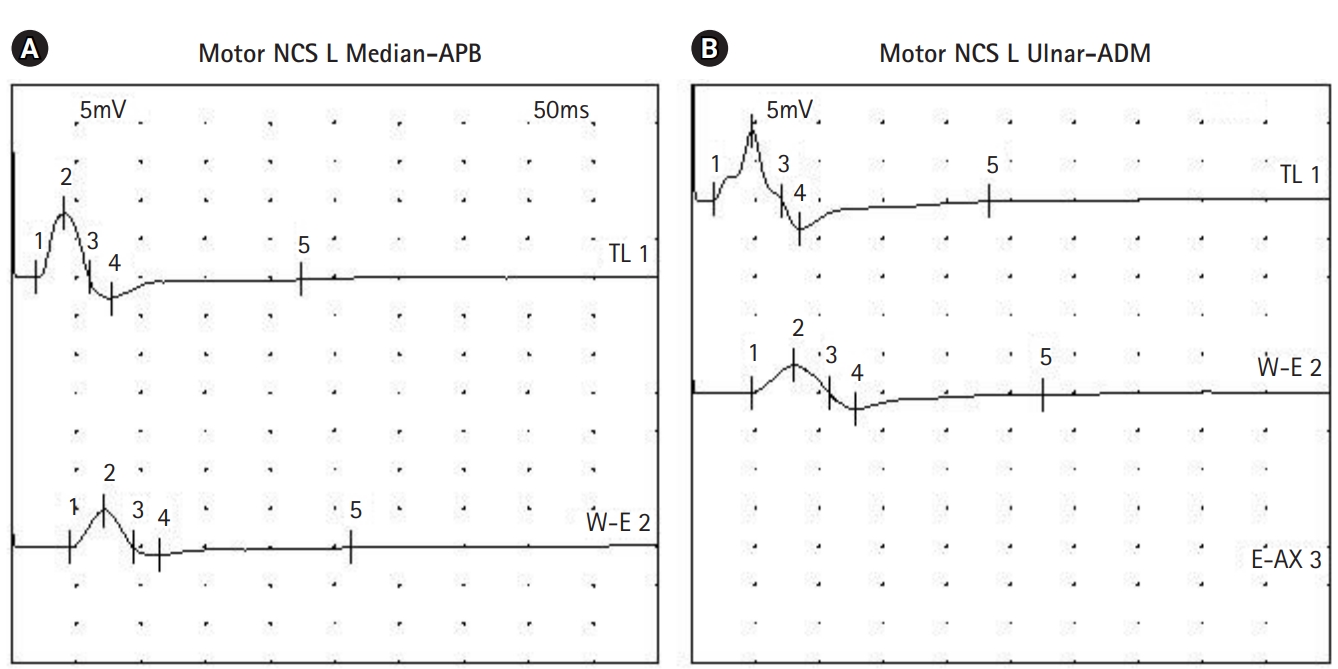
An 8-month-old boy visited our clinic complaining of left facial palsy. He could not close his left eye completely and showed drooping of his left lip. He had a history of bloody diarrhea three weeks before the visit. His medical history revealed secundum atrial septal defect and ventricular septal defect, for which he is being followed up at the cardiology clinic. On physical examination, his vital signs were as follows: heart rate of 100 beats per minute, blood pressure of 95/48 mm Hg, body temperature of 36.5┬░C. His motor power was Medical Research Council (MRC) grade 5 in all extremities, tone was normal, and deep tendon reflexes (DTRs) were normoreflexic in the biceps, knee, and ankle jerks. No pathologic reflexes were present. Routine laboratory blood tests showed a normal complete blood count including the differential, and normal renal and liver function. Serum immunoglobulin M antibodies to Epstein-Barr virus, herpes simplex virus, and mycoplasma were negative. Stool tests for leukocyte, blood, and culture were negative. Stool polymerase chain reaction for Clostridium difficile toxin B and Clostridium perfringens were positive. The patient was diagnosed with idiopathic peripheral facial palsy. He was started on oral steroids. His facial palsy improved, and he was able to close his eyes while sleeping. However, on the 5th day after onset, he developed motor weakness in his legs. His motor strength was MRC grade 3 in the upper extremities and 1 in the lower extremities. DTRs were absent in the biceps, knee, and ankle jerks bilaterally. Cerebrospinal fluid analysis showed 1 white blood cell/┬ĄL with a protein level of 40.2 mg/dL. Antibodies to disialoganglioside (GD1b) and monosialoganglioside (GM1) were negative. Brain magnetic resonance imaging was unremarkable. GBS was diagnosed based on the clinical features, and a 5-day course of intravenous immunoglobulin was started from the day of onset of the motor weakness. On the 6th day, he was reported to have poor sucking ability with a gurgling sound when feeding, and also reduced facial muscle expressions bilaterally. Nerve conduction study was performed on the 8th day after symptom onset. Sensory responses of both upper and lower limb nerves were normal. Motor responses of the left median and ulnar nerve showed a conduction block consistent with a demyelinating neuropathy (Fig. 1). From the 8th day, he was able to move his toes, and swallowing improved. Since then, his neurological condition gradually improved. On the 13th day, he could close both eyes while sleeping. He was discharged with rehabilitative treatment. On the 23rd day, his motor power recovered to MRC grade 5. On the 65th day, DTRs were elicited in the biceps and knee jerks. During the 2-year follow-up, he remained healthy without any neurologic sequelae.
The possible mechanism for facial palsy in GBS is thought to be secondary to the direct attack of antibodies, either causing demyelination or axonal degeneration, depending on the type of antibody involved. Hypertension may contribute to facial paralysis; edema or hemorrhage within the facial canal could cause neural compression [2].
Our case herein showed a very unique presentation of GBS. As mentioned before, unilateral facial palsy in GBS is rare, especially in children. Only a few pediatric case reports have been published [3-5]. Facial palsy in GBS usually follows limb weakness or at least presents simultaneously with other clinical features of GBS such as limb weakness, dysesthesia, and multiple cranial palsies. Presentation of facial palsy alone as the first symptom of GBS is rare [2-4]. There has been one case report of an 11-year-old girl presenting with unilateral facial palsy preceding motor weakness by three days, which is similar to our case [5]. Our patient developed bilateral incomplete facial palsy with bulbar palsy while unilateral facial palsy was improving, which showed a different clinical course from the previous pediatric cases [2-5]. He had no autonomic dysregulations such as labile hypertension, implicating his facial palsy not being related to hypertension.
Anti-GM2 with previous Campylobacter jejuni infection has been linked to bilateral facial paralysis. Iqbal et al. [3] reported a case of an adolescent girl with GBS who presented with unilateral facial palsy after C. jejuni infection, who was positive for anti GM1 and GM2 antibodies. Although our patientŌĆÖs stool was positive for C. difficile toxin B and C. perfringens, we could not be certain about the relationship between unilateral facial palsy and the pathogens isolated from the stool. We could not check for anti GM2 antibodies either, which might have given more clues regarding the development of unilateral facial palsy with GBS in this infant.
The facial palsy reported with GBS is usually bilateral. However, it can rarely present unilaterally, which could be misdiagnosed as Bell palsy. Therefore, pediatricians should know about the association between unilateral facial paralysis and GBS. Written informed consent by the patients was waived due to a retrospective nature of our study.
Notes
Author contributions
Conceptualization: KHL. Data curation: HRN. Formal analysis: HRN and KHL. Methodology: HRN and KHL. Project administration: KHL. Visualization: HRN and KHL. Writing-original draft: HRN. Writing-review & editing: HRN and KHL.
Acknowledgments
This study was presented as a poster at the 68th Annual Autumn Meeting of the Korean Pediatric Society, 2018.
References
2. Verma R, Chaudhari TS, Giri P. Unilateral facial palsy in Guillain-Barre syndrome (GBS): a rare occurrence. BMJ Case Rep 2012 Oct 19 [Epub]. https://doi.org/10.1136/bcr-2012-007077

3. Iqbal M, Sharma P, Charadva C, Prasad M. Rare encounter of unilateral facial nerve palsy in an adolescent with Guillain-Barr├® syndrome. BMJ Case Rep 2016 Jan 28 [Epub]. https://doi.org/10.1136/bcr-2015-213394

- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 3,724 View
- 171 Download
- Related articles in Ann Child Neurol
-
Hemiplegic Migraine Presenting with Unilateral Facial Palsy: A case report2018 December;26(4)
A Child with Guillain-Barr├® Syndrome Presenting Paralytic Ileus.2017 September;25(3)