|
 |
- Search
| Ann Child Neurol > Volume 27(1); 2019 > Article |
|
Abstract
Purpose
This study aimed to identify the clinical characteristics and risk factors of post-traumatic seizure (PTS) in preschool-aged children.
Methods
This study is based on a retrospective electronic medical record review of 1,576 children under 6 years old, who visited our hospital by head trauma from January 1, 2011 to December 31, 2015. We reviewed demographics, causes of head trauma, radiologic findings, Glasgow Coma Scale (GCS) score, and characteristics of seizure. PTS was divided into 3 groups of immediate (within the day of head trauma), early (within 7days) and late (after 7days) seizures.
Results
Of the 1,576 head traumas, 3.4% developed PTS of which 32.1% occurred immediately, 11.3% early, 56.6% lately. The mean age was 2.02±1.63 years and 60.6% was male, 2.6% had fever at the time of visit, and 2.9% had a history of seizures. The causes of head injuries were blunt trauma (34.5%), fall down (29.5%), slip down injury (25.1%), passenger traffic accidents (7.2%), pedestrian traffic accident (1.9%), and causes unknown (1.8%). The severity of traumatic brain injury (TBI) was mild in 99.0%, moderate in 0.4%, and severe in 0.5%. On radiologic findings, 88.6% was normal, 6.0% had skull fracture, 2.8% had intracranial hemorrhage (ICH) and 2.7% had both skull fracture and ICH.
Childhood head trauma is a major cause of death and disability. In the United States, the incidence of head trauma in childhood has increased steadily over the last decade, with more prevalence among children under 4 years of age [1]. In Korea, 55% of head trauma in children under 18 years old occurred in 0 to 4 years old [2]. Posttraumatic seizure (PTS) is complication that occur in patients with head trauma, which is classified according to the latency, immediate seizures within 24 hours after trauma, early seizures within 7 days, and late seizures after 7 days. The overall incidence of PTS in children is 5% to 10% [3-5], and the incidence of PTS in severe traumatic brain injury (TBI) is 10 times higher than in mild TBI [6,7]. Risk factors associated with the PTS include skull fracture, brain parenchymal hemorrhage, focal neurologic deficit at admission, duration of unconsciousness over 24 hours, extensive brain contusion, low Glasgow Coma Scale (GCS) score, abnormal findings in brain computed tomography (CT) scan, and age of 2 years or less [8-13]. Although there were many studies about PTS in children of various ages, there is a lack of research on the clinical characteristics and risk factors of PTS in preschool-aged children.
The purpose of this study was to investigate the incidence, clinical features, and risk factors of PTS in preschool-aged children with head trauma.
From January 2011 to December 2015, total 1,578 patients under 6 years of age visited the emergency department or outpatient clinic of Wonju Severance Christian Hospital due to head trauma. Two patients were excluded due to insufficient medical records and 1,576 patients were enrolled in this retrospective study.
Head trauma is defined as any physical injury to the head. And TBI is defined as a disruption in the normal function of the brain that can be caused by a bump, blow, or jolt to the head or a penetrating head injury [14]. In this study, the causes of the accident were classified into fall down injury, slip down injury, blunt trauma, passenger traffic accident, pedestrian traffic accident and cause unknown. Altered brain function was confirmed by thorough neurologic examination, checking alteration of consciousness, GCS score, focal neurologic deficit. In this study, patients with GCS score 13 to 15 were classified as mild TBI, GCS 9 to 12 as moderate TBI, GCS <8 as severe TBI [15]. PTS are the types of seizures that arise from TBI due to physical trauma [16]. PTSs are commonly derived into three classes by latency; immediate seizures, early seizures, and late seizures. Immediate seizures refer to PTSs that happen at or minutes after impact; PTSs that occur one week after head injury are called early seizures, and late PTSs are defined as those occurring 1 week after injury [12,17,18]. Skull X-rays and brain CT scans were performed if indicated according to Pediatric Emergency Care Applied Research Network (PECARN) rule or if parents wanted.
The characteristics of the patients with and without seizures were compared by t-test at age and chi-square test at the other variables. Cells with an expected frequency of 5 or less were compared using Fisher's exact test. Chi-square test was used to compare the incidence of generalized seizures, and the Kruskal-Wallis test was used to compare the mean latency and duration of seizures. If P value was less than 0.05, it was judged to be statistically significant, and SPSS version 23.0 (IBM Co., Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) were used.
Among the patients with head trauma, 955 (60.6%) were males and 621 (39.4%) were females. The male to female ratio was 1.54:1 and the mean age was 2.02 years. The incidence of head trauma was 388 (24.6%) at 1-year-old, 340 (21.6%) at 0-year-old, 264 (16.8%) at 2-year-old, 203 (12.9%) at 4-year-old, and 155 (9.8%) at 5-year-old by age. PTS occurred in 53 patients (3.36%) (Table 1).
Among the patients with PTS, 17 (32.1%) were immediate seizures, six (11.3%) were early seizures, and 30 (56.6%) were late seizures. The median latency was 0 hours (range, 0 to 8) in immediate seizure, median 2.5 days (range, 2 to 5) in early seizure, and median 197 days (range, 10 to 2,038) in late seizure. Generalized seizure was observed in 20 patients, which was 23.5% in immediate seizures, 50.0% in early seizures, and 43.3% in late seizures. The duration of seizure was 185.0±162.5 seconds in immediate seizure, 570±927.0 seconds in early seizure, and 215.4±186.5 seconds in late seizure (P=0.796) (Table 2).
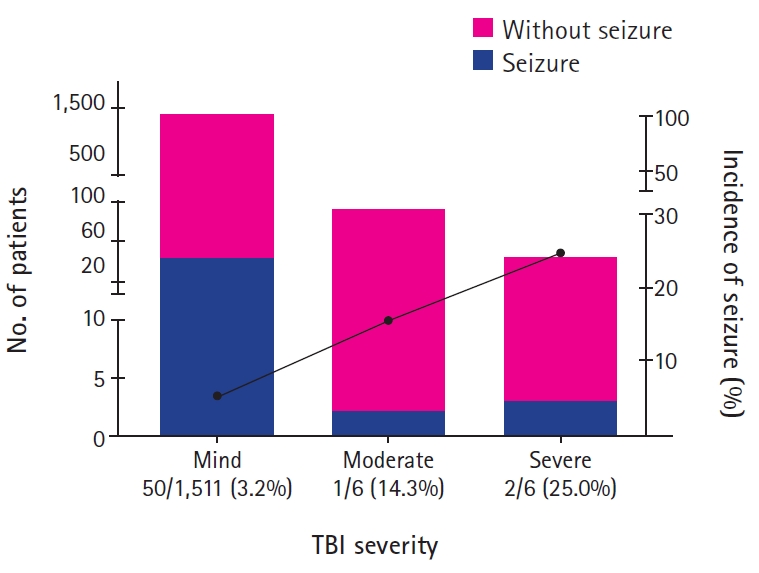
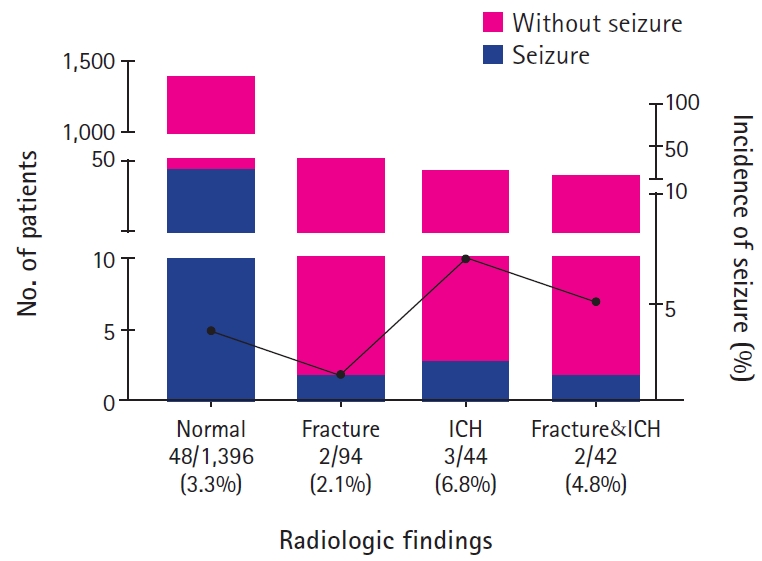
At the time of admission, body temperature was measured in 1,447 patients, and 38 patients (2.6%) had fever over 38.0℃. Nine of 38 patients (23.7%) with fever, and 38 of 1,409 patients (2.7%) without fever experienced PTS (odds ratio [OR], 11.20; P<0.001). There was a history of seizures in 45 of patients (2.86%) with head trauma, and 10 of them (22.2%) had PTS, while only 43 of 1,531 patients (2.8%) without history of seizures had PTS (OR, 9.22; P<0.001). The causes of head trauma were in the order of blunt trauma (34.5%), fall down injury (29.5%), slip down injury (25.1%), passenger traffic accident (7.2%), pedestrian traffic accident (1.9%). The incidence of PTS was highest (4.8%) in slip down injury (P=0.27). According to the TBI severity by GCS scores, 1,561 patients (99.0%) had mild TBI, seven patients (0.4%) had moderate TBI, and eight patients (0.5%) had severe TBI. The incidence of PTS was 3.2% in mild TBI, 14.3% in moderate TBI, and 25.0% in severe TBI (P=0.012) (Fig. 1). Skull X-ray and brain CT scan results were normal in 1,396 (88.6%), skull fracture in 94 (6.0%), intracranial hemorrhage (ICH) in four (2.8%), concurrent skull fracture and ICH in 42 (2.7%), and it was not correlated with the incidence of seizures (P=0.40) (Table 1 and Fig. 2).
It is known that PTSs occur in 5% to 10% of children, and the incidence recently reported in Korea was 12.3%, among which early seizure was 70.7% and late seizure was 29.3% [19]. It has been reported that about 60% of early seizures develop immediately within 24 hours after head trauma [3], which is called ‘immediate seizure.’ In this study, PTS occurred 3.36% in preschool-aged children, among them, 43.4% of early seizures and 56.6% of late seizures occurred and 73.9% of early seizures showed immediate seizures. In previous studies, the study group was set up as an inpatient rather than an emergency room or an outpatient. In this study, however, the incidence may have been lowered because the number of patients who discharged was included in the study population. According to Asikainen et al. [5], the risk of early PTS is high in children younger than 7 years and the risk of late PTS is high in adolescents and adults, but in this study, the incidence of late seizures was higher than early seizures in children under 6 years of age. This conflicting result is likely to be from the difference in inclusion criteria. In this study, patients with seizure with fever, with previous history of seizure attack, with a history of epilepsy were all included while most of previous studies excluded them. Because the current definition of PTS is not clear about the history of fever, previous seizures or epilepsy, discussions about more clear definition will be needed in the future.
The latency of PTS is known as an average of 2 years, and in the case of early seizure, 25% of the cases appear within 1 hour after trauma and 50% of the cases occur within 24 hours after trauma [4,6,20,21]. In this study, the overall latency was average of 7.2 months, with an average of 1 hour in immediate seizure, 3.0 days in early seizure, and 15.0 months in late seizure.
According to Youn et al. [19], 82.9% of generalized seizures, 17.1% of partial seizures, and 2.4% of status epilepticus were observed in early PTS, while 76.5% of generalized seizures and 23.5% of partial seizures were observed in late PTS. However in this study, 28.6% of generalized seizures were observed in immediate PTS patients, 50.0% in early PTS, and 44.8% in late PTS. It is known that in posttraumatic epilepsy patients, the seizure usually occurs in the mesial temporal lobe or frontal lobe, and not frequently from occipital or parietal lobe [22]. Although PTS can occur in all forms, most early PTS show a pattern of generalized tonic-clonic seizures [23], about two-thirds of late PTS show generalized seizures or partial seizure with secondary generalization [24-28]. In this study, the incidence of generalized or partial seizures was analyzed but it was not analyzed for more detailed appearance of seizures, presence of secondary generalization, the relationship between PTS and electroencephalography (EEG) or radiologic findings, so further analysis will be needed.
Mean duration of PTS was 4.2 minutes, which was 3.1 minutes for immediate PTS, 9.5 minutes for early PTS, and 3.6 minutes for late seizure. The duration was the longest in early PTS group except immediate seizure, but the duration of PTS in children has not been studied yet.
This study was the first which analyzed the risk factors of PTS in preschool-aged children, under 6 years. In this study, the risk factors for PTS were fever over 38.0℃ at the time of head trauma, history of seizure, and low GCS score at visit. However, age, sex, cause of head trauma, and radiologic findings were not associated with PTS. Because there is little known about the relationship between fever and PTS, it is noteworthy that fever was identified as a risk factor for PTS, especially considering that fever is frequently observed in preschool-aged children. Seizures with fever after head trauma may be difficult to distinguish from febrile seizures, which are common in this age group. Therefore, additional studies such as EEG, brain imaging modalities, and close follow-up are necessary.
Previous studies have shown that low GCS score in head trauma patients is a poor prognostic factor for TBI and is a risk factor for PTS [29-31]. Thapa et al. [32] reported that the risk of PTS in severe TBI increased about four times in patients with a GCS score of less than 9, compared to patients with a GCS score of 14 to 15. Similarly, in this study, the risk of PTS was 7.8 times higher in patients with severe TBI compared to mild TBI.
In children less than 2 years of age, the skull is thin due to its anatomical structure and it is vulnerable to fractures. In addition, the subarachnoid space is narrow so that TBI risk increases. For these reasons, PTS in this age group is relatively common, especially early PTS [33-35]. In this study, PTS occurred most frequently at 1 year of age (5.2%), but it was not significant.
In this study, 60.6% of the patients with head trauma were male and there was no difference in incidence of PTS according to sex, same as our previous study [36].
The causes of childhood head trauma in Korea during the last 10 years are as follows. For children under 2 years of age in 2007, 47.4% of fall down injury, 19% of crash injury, 9.5% of traffic accidents were reported [37], and for prechool-aged children in 2015, 71.4% of fall down injury, 22.1% of crash injury, 6.5% of traffic accident were reported [36]. And in this study, the causes of head trauma in children under 6 year of age were 34.5% of blunt trauma, 29.5% of fall down injury, 25.1% of slip down injury, 7.1% of passenger traffic accident, 1.9% of pedestrian traffic accident, and 1.8% of unknown cause. Because ‘blunt trauma’ and ‘slip down injury’ are type of collision, it may be thought that fall down injuries has been decreased while injury by collision has increased recently.
Kim et al. [38] reported 63.2% of cerebral concussion and 45.6% of skull fracture in children under 2 years of age according to radiologic findings. Kim et al. [39] reported that 83.8% of PTS patients under 18 years of age had radiographic abnormalities, of which 51.6% had brain parenchymal hemorrhage and 45.1% had ICH. In this study, radiologic diagnosis of head trauma patients under the age of 6 years was similar to that of the previous study in the order of brain contusion (88.6%), skull fracture (8.8%), ICH (5.5%).
The purpose of this study was to investigate the incidence, clinical features and risk factors of PTS in children under 6 years old. It is the largest study in Korea, and has identified some interesting results presented above, but it has some limitations. First, because it was a retrospective study based on medical records, there was a limitation in getting more detail history of head trauma, associated symptoms and in evaluation of TBI severity. Also we could not analyze the prognosis because of the lack of long-term follow-up. Second, because the results of this study are based on data collected from a single institution in a city, it is difficult to generalize these results as the clinical features of head injury and PTS in preschool-aged children in Korea. In the future, a randomized controlled trial in larger population is needed to know the clinical characteristics, risk factors, and the prognosis of pediatric head trauma and PTS in Korea.
Table 1.
Comparison of clinical characteristics between seizure and non-seizure group
Table 2.
Characteristics of immediate, early, and late posttraumatic seizure
References
1. Centers for Disease Control and Prevention. National hospital ambulatory medical care survey: 2010 emergency department summary tables. Atlanta: Centers for Disease Control and Prevention; 2013.
2. Kim HB, Kim DK, Kwak YH, Shin SD, Song KJ, Lee SC, et al. Epidemiology of traumatic head injury in Korean children. J Korean Med Sci 2012;27:437-42.



5. Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia 1999;40:584-9.


7. Annegers JF, Grabow JD, Groover RV, Laws ER Jr, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology 1980;30(7 Pt 1):683-9.


8. Pohlmann-Eden B, Bruckmeir J. Predictors and dynamics of posttraumatic epilepsy. Acta Neurol Scand 1997;95:257-62.


9. Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology 1985;35:1406-14.


10. Hahn YS, Fuchs S, Flannery AM, Barthel MJ, McLone DG. Factors influencing posttraumatic seizures in children. Neurosurgery 1988;22:864-7.



12. Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia 2003;44(s10):11-7.

13. Ratan SK, Kulshreshtha R, Pandey RM. Predictors of posttraumatic convulsions in head-injured children. Pediatr Neurosurg 1999;30:127-31.


14. Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta: Centers for Disease Control and Prevention; 2010. p. 891-904.
15. Valadka AB, Narayan RK. Emergency room management of the head-injured patient. In: Narayan RK, Wilberger JE, Povlishock JTet al. editors. Neurotrauma. New York: McGraw-Hill; 1996. p. 122.
16. Wagner AK, Miller MA, Scanlon J, Ren D, Kochanek PM, Conley YP. Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res 2010;90:259-72.



18. Jennett WB. Epilepsy after non-missile head injuries. 2nd ed. London: Heinemann Medical; 1975.
19. Youn S, Lee MA, Kim HM, Cha BH. The incidence and risk factors of posttraumatic seizure in children. Korean J Pediatr 2004;47:1198-204.
20. Caveness WF, Meirowsky AM, Rish BL, Mohr JP, Kistler JP, Dillon JD, et al. The nature of posttraumatic epilepsy. J Neurosurg 1979;50:545-53.


21. Pagni CA. Posttraumatic epilepsy. Incidence and prophylaxis. Acta Neurochir Suppl (Wien) 1990;50:38-47.


22. Ding K, Gupta PK, Diaz-Arrastia R. Epilepsy after traumatic brain injury. In: Laskowitz D, Grant Get al. editors. Translational research in traumatic brain injury. Boca Raton: CRC Press; 2016. p. 299-314.
23. Engel J, Pedley TA, Aicardi J. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1998.
24. Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil 1997;78:835-40.


25. Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil 2003;84:365-73.


26. Mazzini L, Cossa FM, Angelino E, Campini R, Pastore I, Monaco F. Posttraumatic epilepsy: neuroradiologic and neuropsychological assessment of long-term outcome. Epilepsia 2003;44:569-74.


27. De Reuck J. Risk factors for late-onset seizures related to cerebral contusions in adults with a moderate traumatic brain injury. Clin Neurol Neurosurg 2011;113:469-71.


28. Kazemi H, Hashemi-Fesharaki S, Razaghi S, Najafi M, Kolivand PH, Kovac S, et al. Intractable epilepsy and craniocerebral trauma: analysis of 163 patients with blunt and penetrating head injuries sustained in war. Injury 2012;43:2132-5.


29. Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19:869-74.


30. McNett M. A review of the predictive ability of Glasgow Coma Scale scores in head-injured patients. J Neurosci Nurs 2007;39:68-75.


31. Saadat S, Akbari H, Khorramirouz R, Mofid R, Rahimi-Movaghar V. Determinants of mortality in patients with traumatic brain injury. Ulus Travma Acil Cerrahi Derg 2012;18:219-24.


32. Thapa A, Chandra SP, Sinha S, Sreenivas V, Sharma BS, Tripathi M. Post-traumatic seizures: a prospective study from a tertiary level trauma center in a developing country. Seizure 2010;19:211-6.


33. Berney J, Favier J, Froidevaux AC. Paediatric head trauma: influence of age and sex. I. Epidemiology. Childs Nerv Syst 1994;10:509-16.


34. Bishop DV. Plasticity and specificity of language localization in the developing brain. Dev Med Child Neurol 1981;23:251-5.


35. Duhaime AC, Alario AJ, Lewander WJ, Schut L, Sutton LN, Seidl TS, et al. Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 1992;90(2 Pt 1):179-85.



36. Na YH, Koh ID, Cha BH. The risk factors of brain injury by head trauma in preschool aged children. J Korean Child Neurol Soc 2015;23:153-8.

37. Jeong JI, Kim AJ, Shin DW, Rho JY, Kim KH, Kim HY, et al. The clinical usefulness of halo sign on CT image of trauma patients. J Korean Soc Traumatol 2007;20:83-9.
38. Kim JK, Park JY, Cho TH, Kwon TH, Lim DJ, Chung YK, et al. Clinical features and prognostic factors of head injury in less than two-year-old children. J Korean Neurosurg Soc 1998;27:625-31.
39. Kim JS, Ryu HW, Byun SH, Kim H, Lim BC, Chae JH, et al. Clinical characteristics of post-traumatic seizures in children. J Korean Child Neurol Soc 2012;20:228-33.
- TOOLS
-
METRICS

- Related articles in Ann Child Neurol
-
Clinical Features of Moyamoya Disease in Children.2007 November;15(2)
Clinical Review of the of Seizures among Children.2010 November;18(2)
Clinical Features of Seizures Related to Rickets in Breastfed Children.2012 September;20(3)
Clinical Characteristics of Post-traumatic Seizures in Children.2012 December;20(4)