|
 |
- Search
| Ann Child Neurol > Volume 32(2); 2024 > Article |
|
Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that affects the overall cognitive, emotional, social, and physical health of the affected individual. It is characterised by challenges in social communication and interaction, repetitive and stereotyped behaviours, and narrow interests. The pathogenesis of ASD is thought to involve a combination of genetic and environmental factors. Increasing evidence suggests that vitamin D deficiency during pregnancy and early childhood may contribute to the development of ASD. While studies have indicated that vitamin D supplementation can significantly improve symptoms of ASD, the underlying mechanism remains elusive. This review summarises the association between vitamin D levels and ASD, explores potential mechanisms underlying vitamin D's role in ASD, and examines the effect of vitamin D supplementation on ASD symptoms.
Autism spectrum disorder (ASD) encompasses a range of neurodevelopmental disorders characterised by challenges in social interaction and communication, restricted interests, repetitive and stereotypical behaviours, and sensory processing issues that typically appear in early childhood [1]. The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, published by the American Psychiatric Association, classifies childhood autism, pervasive developmental disorder not otherwise specified, and Asperger's syndrome under the umbrella of ASD [2]. Genetic factors are believed to contribute to 10% to 20% of ASD cases [3]. Potential environmental factors include advanced parental age, birth by caesarean section, exposure to lead, maternal obesity, hypertension, air pollution, and diabetes [4-8].
Vitamin D is widely recognised for its immunomodulatory properties. The active form of vitamin D, calcitriol, can bind to vitamin D receptors (VDRs) and modify gene transcription. These VDRs are expressed by all immune cells in the human body, enabling vitamin D to carry out a range of functions [9]. It is estimated that 30% of the global population is deficient in vitamin D, while 60% have suboptimal levels [10]. Recent discoveries have highlighted the importance of vitamin D in the development of the foetal and early postnatal brain, beyond its established role in regulating calcium and phosphorus levels. An increasing amount of evidence indicates that vitamin D is implicated in the pathogenesis of ASD, and that vitamin D deficiency may be a contributing factor to ASD [11]. Concurrently, some studies have demonstrated that vitamin D can ameliorate the core symptoms in children with ASD [12]. This review summarises the relationship between vitamin D levels and ASD, explores the potential mechanisms by which vitamin D may be involved in ASD, and discusses the impact of vitamin D supplementation on ASD.
ASD was once considered a rare condition; however, its prevalence has surged in recent decades across various countries [13]. The World Health Organisation reports a global prevalence of ASD at 0.76%, but this figure represents about 16% of the global child population [14]. The Centres for Disease Control and Prevention indicate that, in the United States, the prevalence of autism among 8-year-old children was one in 54 in 2016, with a male-to-female ratio of 4.3:1 [15]. Although there are no national data on the ASD epidemic in China, a multicentre study suggests that the prevalence of ASD among children aged 6 to 10 years is approximately 1% in China, which points to an upward trend [16]. ASD impacts children across all racial, ethnic, and socioeconomic backgrounds, yet the rates of diagnosis vary significantly. Caucasian children are diagnosed with ASD more frequently than black or Hispanic children [17]. Studies have also found that ASD is more prevalent in urban areas compared to rural regions. This is attributed to factors such as rainy and cloudy weather, reduced exposure to ultraviolet B (UVB) rays, and higher levels of air pollution, all of which diminish ultraviolet radiation at the Earth's surface and, consequently, vitamin D synthesis in the skin [18-20]. These findings lend support to the aetiological theory that a deficiency in vitamin D may contribute to the development of ASD.
ASD is a neurobiological disorder shaped by a combination of genetic and environmental factors that influence brain development [21]. Although there have been relatively few neuropathological studies, findings have identified abnormalities in the limbic system, differences in cerebellar structure and connectivity, and alterations in the cortical areas of the frontal and temporal lobes, along with other subtle malformations [22-24]. A small-scale exploratory study examining the neocortical architecture of young children indicated a focal disruption in the cortical laminar structure in the majority of participants, suggesting problems with the formation of cortical layers and neuronal differentiation [25]. Brain overgrowth, characterised by both an increase in cortical size and a rise in extra-axial fluid, has been observed in children with ASD. This phenomenon is an area of ongoing research, aimed at improving our understanding of its origins and investigating its potential as a biomarker [26,27].
Siblings of individuals with ASD exhibit an increased risk of receiving a diagnosis than the general population, and identical twins demonstrate a significantly higher—but not guaranteed—rate of concordance for an ASD diagnosis [28-30]. ASD is associated with genes that produce proteins crucial for neuronal synapses or those involved in neurons’ activity-dependent changes, such as transcription factors [28,31]. Epigenetic alterations, including DNA methylation and histone acetylation, as well as disruptions in transcription and splicing, may also contribute to the condition [28,32-34]. ASD is one of the most genetically diverse neuropsychiatric disorders, with rare de novo and inherited variants identified in over 700 genes [35]. The manifestation of genetic predispositions in ASD exhibits a broad spectrum [36]. Environmental factors occurring before, during, and after birth may also modulate the genetic susceptibility in some individuals [37]. Increased risks of having a child with ASD have been associated with both older maternal and paternal ages [38]. While a maternal history of autoimmune diseases, such as diabetes, thyroid disorders, or psoriasis, has been suggested as a potential risk factor, research findings have been inconsistent [39,40]. Recent studies have highlighted maternal infection or immune activation during pregnancy as another area of interest, potentially representing a risk factor [41-43]. The risk for ASD has been associated with both shorter and longer intervals between pregnancies [44]. Additionally, premature infants face a heightened risk for ASD and other neurodevelopmental disorders [45]. Obstetric complications such as preeclampsia, preterm birth, caesarean delivery, low birth weight, uterine bleeding, and low Apgar scores have been identified as some of the more consistently associated factors with autism in previous epidemiological reviews [46].
Vitamin D is a fat-soluble vitamin encompassing a group of steroid-like substances, including ergocalciferol (D2) and cholecalciferol (D3) [47]. This essential nutrient is synthesised in the skin when 7-dehydrocholesterol interacts with UVB radiation [48]. Subsequently, it undergoes two hydroxylation processes in the liver and kidneys to form 1,25(OH)D and then 1,25(OH)2D3, which activates VDRs to elicit a biological response. Due to the strict regulation of 1,25(OH)2D3 synthesis, serum 25-(OH)D is considered the most reliable indicator of vitamin D status. Traditionally, vitamin D has been recognised for its role in regulating calcium and phosphorus metabolism, which is vital for bone growth and development [49]. However, recent studies have revealed that the enzyme crucial for vitamin D synthesis, 1α-hydroxylase, along with VDRs, is prevalent in brain tissue, indicating that vitamin D is also crucial for brain development [50]. Vitamin D contributes to brain development and function by modulating synaptic plasticity, influencing the dopaminergic system, and reducing oxidative stress [51]. Furthermore, research suggests that vitamin D3 may enhance the formation of regulatory T-cells, thereby preventing excessive immune responses and autoimmune conditions [52].
Increasingly many researchers are examining changes in serum vitamin D levels in ASD children. Numerous studies on the vitamin D status of children and adolescents with ASD from multiple regions and backgrounds have found that ASD children and adolescents had lower vitamin D levels [53-59]. Studies have revealed that vitamin D concentrations in children with ASD are not only lower than those in control groups, but also that there is a significant negative correlation between vitamin D levels and total scores on various assessment tools, including the Social Responsiveness Scale (SRS), Autism Treatment Evaluation Checklist (ATEC), ATEC's social affect domain, Autism Behaviour Checklist (ABC), the behavioural affect domain, and the Childhood Autism Rating Scale (CARS). This correlation suggests that lower vitamin D levels are associated with increased severity of autism symptoms [60,61]. Furthermore, a meta-analysis has confirmed that children and adolescents with ASD have significantly lower levels of vitamin D than participants in control groups [12].
There is evidence suggesting a link between maternal 25-(OH)D levels during pregnancy and neurodevelopmental outcomes in children. Specifically, children whose mothers had low prenatal 25-(OH)D levels (less than 20 ng/mL) exhibited more symptoms associated with ASD, faced greater cognitive challenges, and demonstrated poorer social skills by the age of 5 [62]. One study found that mothers with ASD had significantly lower maternal serum concentrations of 25-(OH)D than mothers without ASD, with 55.9% and 29.4% of them experiencing vitamin D deficiency, respectively [63]. Furthermore, reduced serum levels of 25-(OH)D in the first trimester, as well as low maternal vitamin D levels in the second trimester and at birth, have been associated with a significantly increased risk of ASD in offspring [64]. Additionally, it has been shown that low vitamin D levels during pregnancy and limited exposure to solar UVB radiation may elevate the risk of ASD [65]. Higher prenatal 25-(OH)D levels positively affect cognitive development in offspring, and 25-(OH)D levels in early pregnancy may exert a stronger impact on neurodevelopment than levels later in pregnancy [49].
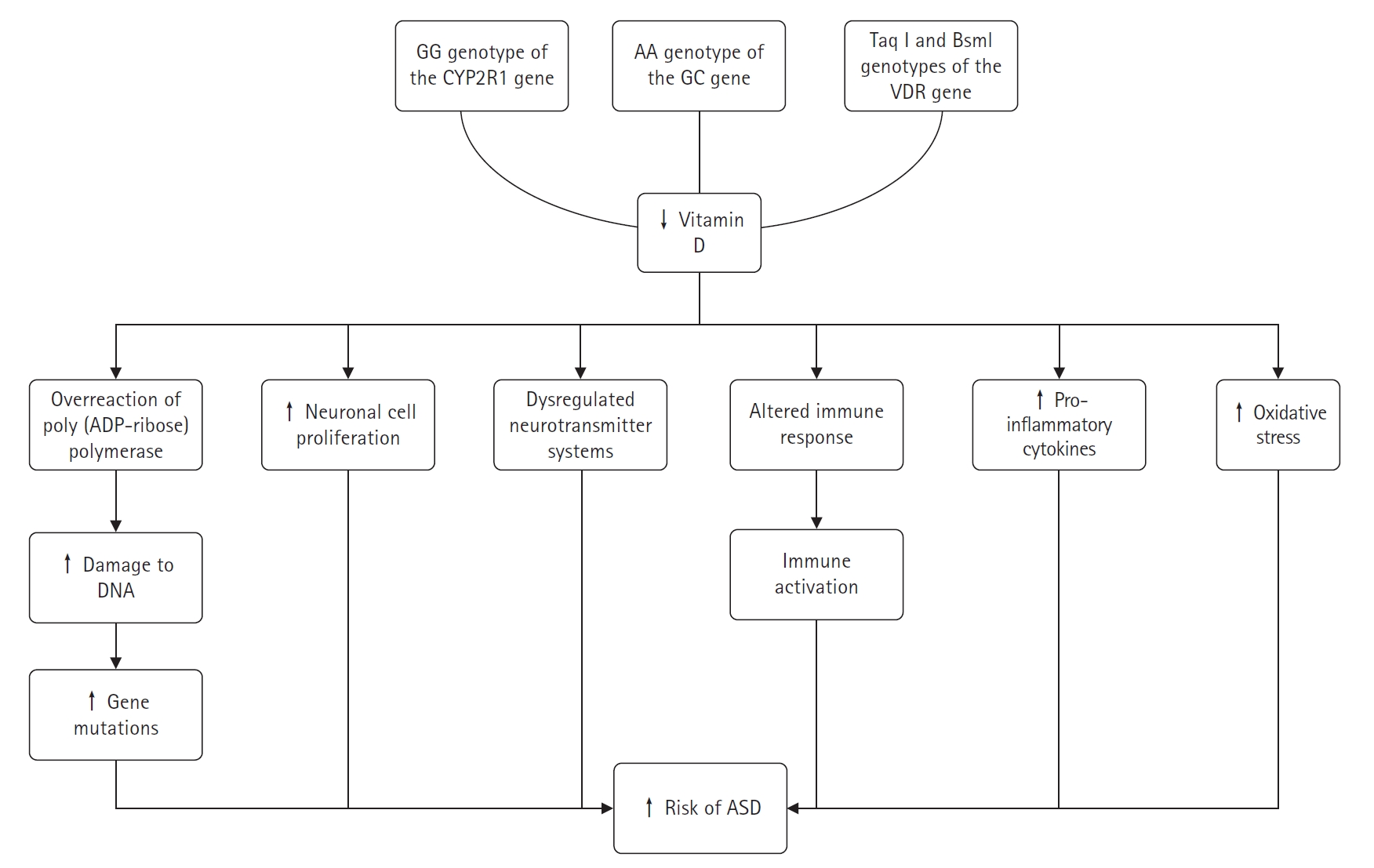
It was found that children with the GG genotype of the cytochrome P450 family 2 subfamily R member 1 (CYP2R1) gene (encoding an enzyme that catalyses the conversion of vitamin D to 25-(OH)D), the AA genotype of the GC gene (encoding a vitamin D-binding protein), and the paternal Taq l and Bsml genotypes of the VDR gene had an increased risk of ASD, emphasising the possible aetiological role of low vitamin D concentrations in ASD [66]. Recent genetic studies on ASD have identified numerous neonatal mutations in affected children [25]. Vitamin D is capable of DNA repair and maintenance through several mechanisms. To date, over five vitamin D-dependent genes have been identified that encode proteins involved in the permanent repair of DNA mutations [67]. Vitamin D has the ability to repair and maintain DNA through a variety of mechanisms. Low vitamin D levels can lead to an overactivation of the DNA repair enzyme poly(adenosine diphosphate ribose) polymerase, which may inadvertently damage adjacent DNA. Daily supplementation with low doses of vitamin D3 has been shown to prevent gene mutations, enhance apoptosis, and increase Bax levels [68]. Research has established that several proteins closely associated with vitamin D, such as growth arrest and DNA-damage-inducible alpha, RAD23 homolog B, and poly(adenosine diphosphate ribose), play roles in DNA damage repair [25,67-69]. Consequently, vitamin D deficiency may contribute to gene mutations in children with ASD.
When vitamin D levels are insufficient, neuronal cells may proliferate excessively, leading to increased brain growth during early development stages, which could be associated with the onset of ASD [70,71]. In neuroanatomy, animal models of developmental vitamin D deficiency have successfully replicated phenotypes associated with ASD [72]. Vitamin D-related neurotransmitters, including 5-hydroxytryptamine, oxytocin, serotonin, dopamine, and γ-aminobutyric acid, are known to regulate learning, memory, and emotions [73-75]. Dysregulation of neurotransmitter systems, such as the oxytocinergic, dopaminergic, and serotonergic systems, is thought to contribute to ASD. These systems are crucial for brain maturation, neurotransmission, behaviour, and cortical organisation [74]. Rodent studies have shown that vitamin D administration can increase gamma-aminobutyric acid (GABA) production in brain regions like the hippocampus, anterior cingulate cortex, and prefrontal cortex [73]. Individuals with ASD have been found to have lower plasma oxytocin levels and abnormal serotonin concentrations in the brain and peripheral tissues [74]. Brain dopamine transporter binding is significantly higher in ASD patients compared to healthy controls, while brain serotonin transporter binding is significantly lower in high-functioning adults with ASD [76]. There is evidence of increased peripheral blood 5-hydroxytryptamine (serotonin) and decreased brain concentrations in individuals with ASD [74].
Immune activation is considered a risk factor for ASD. A deficiency in vitamin D may alter the immune responses of ASD patients and could prevent behavioural issues related to immune activation [76]. Autoimmune markers, such as anti-ganglioside M1 antibodies, anti-nucleosome-specific antibodies, anti-nuclear antibodies, and anti-myelin basic protein autoantibodies, are more prevalent in ASD patients, with their levels positively correlating with ASD severity [64]. Mostafa and Al-Ayadhi [77] found that anti-myelin-associated glycoprotein (anti-MAG) levels were higher in 70% of children with ASD, and there was a significant negative correlation between serum 25-(OH)D levels and anti-MAG levels. This suggests that vitamin D deficiency in some children with ASD may contribute to elevated anti-MAG levels, which could play a role in brain damage associated with ASD. These findings highlight the importance of vitamin D in the development of autoantibodies and the pathophysiology of ASD. According to recent research, ASD is an inflammatory disease [54,55]. Vitamin D is known to have an immunomodulatory effect, enhancing protective immune responses while reducing inflammatory ones [78]. The anti-inflammatory effects of vitamin D on the brain include reducing neurotoxicity, oxidative-induced neuroinflammation, and harmful inflammatory cytokines. Abnormal immune function in people with ASD, such as elevated inflammatory cytokine levels, is similar to that seen in vitamin D deficiency [19]. Pro-inflammatory cytokines like interferon-γ, tumour necrosis factor-α (TNF-α), and interleukin-6 (IL-6) are found in higher concentrations in children with ASD and are associated with cognitive impairment [79,80]. Vitamin D metabolites have been shown to decrease the release of TNF-α and IL-6, increase the expression of anti-inflammatory cytokines like interleukin-10 (IL-10) from activated B-cells, and promote a more tolerogenic state in dendritic cells. The activation of calcitriol leads to lower production of inflammatory cytokines, thereby protecting brain tissue [81].
Vitamin D supplementation is thought to reduce oxidative stress and play a protective role in the brain. Several lines of evidence suggest that oxidative stress and mitochondrial dysfunction are common in ASD [82]. Children with ASD have higher levels of oxidised glutathione in their plasma, which causes a corresponding rise in oxidative stress [83]. Vitamin D is an antioxidant that can increase glutathione levels, decrease glial cell activation, inhibit nitric oxide synthase synthesis, and attenuate neuroinflammation; thus, it plays an important role in neuromodulation and neuroprotection [84].
Neuroanatomical defects in vitamin D-deficient mice include increased brain volume and lateral ventricle size, aberrant brain calcium signalling, and dysfunctional mitochondria. These mice also demonstrate an increased vulnerability to oxidative stress, alterations in dopamine and serotonin signalling that lead to changes in neurotransmission, and a distinctly atypical immune response associated with a higher risk of autoimmune diseases and chronic inflammatory conditions [72]. The behaviour of young children with autism shows similarities to that of vitamin D-deficient animals [85]. Vitamin D supplementation may influence animal models of ASD, and the timing of vitamin D deficiency may determine the presence or absence of ASD-related symptoms in rodent studies [54,86,87]. Fig. 1 summarizes the complex role of vitamin D in the development of ASD.
A prospective study that administered vitamin D to pregnant women who already had children with autism found that only 5% of their new-borns were diagnosed with autism, a figure notably lower than the rates of 20% or higher reported in existing literature [86,88]. However, some researchers caution against drawing firm conclusions from these findings, as the study lacked a control group and involved a relatively small sample of pregnant women with varying lengths of vitamin D supplementation. They contend that the current evidence does not substantiate the claim that vitamin D supplementation during pregnancy can prevent the onset of ASD [89]. In a 2014 study, a 32-month-old boy with ASD and a deficiency in vitamin D received a daily oral dose of 400 IU of vitamin D3 and a monthly intramuscular injection of 150,000 IU of vitamin D3 for 2 months. This treatment led to temporary improvements in the core symptoms of ASD [90]. A case-control study published in 2015 reported that after 6 months of vitamin D supplementation, participants showed improvements in scores on the CARS, the Vineland Adaptive Behaviour Scale, and the ATEC. Although the group that received vitamin D supplements exhibited slight improvements in ASD symptoms, the differences between the supplemented and non-supplemented groups were not statistically significant [91]. Another study indicated that 37 children with ASD and low vitamin D levels (25-(OH)D <75 nm/L) who received vitamin D supplements for 3 months experienced significant enhancements in ASD symptoms, as measured by the ABC and CARS scores [92]. The latest randomised controlled trials and meta-analyses of vitamin D for the treatment of ASD have also shown that vitamin D can improve the core symptoms of ASD [93,94].
The high incidence of ASD has turned it into a pressing social issue, yet the etiology of the disorder remains elusive. While ASD is believed to be influenced by a combination of genetic and environmental factors, existing research has not satisfactorily accounted for the disorder's epidemiological traits, and pharmacological interventions have yet to prove effective. Consequently, exploring the origins and development of ASD from a novel angle is crucial for devising ground-breaking treatment strategies. There is evidence to suggest that insufficient vitamin D levels during pregnancy, after birth, and in early childhood may be associated with neurodevelopmental disorders, including ASD. Certain studies indicate that vitamin D supplementation in vitamin D-deficient children with ASD can ameliorate their fundamental symptoms. To fully comprehend the connection between vitamin D and ASD, extensive research is required. Delving deeper into this relationship could pave the way for a straightforward, cost-effective, and safe new approach to treating and preventing ASD.
Notes
Author contribution
Conceptualization: RWS, TWS, and SWS. writing-original draft: RWS, TWS, and SWS. review & editing: RWS, TWS, and SWS.
References
1. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet 2018;392:508-20.



3. Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 2008;9:341-55.




4. Salhia HO, Al-Nasser LA, Taher LS, Al-Khathaami AM, El-Metwally AA. Systemic review of the epidemiology of autism in Arab Gulf countries. Neurosciences (Riyadh) 2014;19:291-6.


5. Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res 2018;84:190-8.



6. Kim JY, Son MJ, Son CY, Radua J, Eisenhut M, Gressier F, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry 2019;6:590-600.


7. Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism 2017;8:13.




8. National Institute of Environmental Health Sciences. Autism. Research Triangle Park: National Institute of Environmental Health Sciences; 2018.
9. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685-98.




10. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 2017;18:153-65.



11. Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, Sadek B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front Neurosci 2018;12:304.



12. Wang Z, Ding R, Wang J. The association between vitamin D status and autism spectrum disorder (ASD): a systematic review and meta-analysis. Nutrients 2020;13:86.



13. Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. Natl Health Stat Report 2015;87:1-20.
14. Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med 2015;45:601-13.


15. Maenner MJ, Shaw KA, Baio J; EdS1, Washington A, Patrick M, et al. Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ 2020;69:1-12.



16. Sun X, Allison C, Wei L, Matthews FE, Auyeung B, Wu YY, et al. Autism prevalence in China is comparable to Western prevalence. Mol Autism 2019;10:7.




17. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67:1-23.



20. Wai KM, Yu PK, Lam KS. Reduction of solar UV radiation due to urban high-rise buildings: a coupled modelling study. PLoS One 2015;10:e0135562.



21. Steinman G. The putative etiology and prevention of autism. Prog Mol Biol Transl Sci 2020;173:1-34.



22. Johnson CP, Myers SM; American Academy of Pediatrics Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007;120:1183-215.



23. Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, et al. Regional alterations in purkinje cell density in patients with autism. PLoS One 2014;9:e81255.



24. Stoodley CJ, D'Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 2017;20:1744-51.




25. De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014;515:209-15.




26. Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, et al. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry 2017;82:186-93.



27. Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017;542:348-51.




28. Kim H, Keifer C, Rodriguez-Seijas C, Eaton N, Lerner M, Gadow K. Quantifying the optimal structure of the autism phenotype: a comprehensive comparison of dimensional, categorical, and hybrid models. J Am Acad Child Adolesc Psychiatry 2019;58:876-86.



29. Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311:1770-7.



30. Risch N, Hoffmann TJ, Anderson M, Croen LA, Grether JK, Windham GC. Familial recurrence of autism spectrum disorder: evaluating genetic and environmental contributions. Am J Psychiatry 2014;171:1206-13.


31. Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science 2003;302:826-30.


32. Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011;474:380-4.




33. Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry 2014;19:862-71.




34. Sun W, Poschmann J, Cruz-Herrera Del Rosario R, Parikshak NN, Hajan HS, Kumar V, et al. Histone acetylome-wide association study of autism spectrum disorder. Cell 2016;167:1385-97.


35. Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr 2020;9:S55-65.



36. Veenstra-Vanderweele J, Christian SL, Cook EH. Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet 2004;5:379-405.


37. Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore) 2017;96:e6696.



38. Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007;161:334-40.


39. Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK. Maternal type 1 diabetes and risk of autism in offspring. JAMA 2018;320:89-91.



40. Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005;159:151-7.


41. Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 2012;26:607-16.



42. Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 2015;16:469-86.




43. Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016;351:933-9.



44. Schieve LA, Tian LH, Drews-Botsch C, Windham GC, Newschaffer C, Daniels JL, et al. Autism spectrum disorder and birth spacing: findings from the study to explore early development (SEED). Autism Res 2018;11:81-94.




45. Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 2018;142:e20180134.



46. Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health 2007;28:235-58.


47. Grant WB. The role of geographical ecological studies in identifying diseases linked to UVB exposure and/or vitamin D. Dermatoendocrinol 2016;8:e1137400.




48. Bivona G, Gambino CM, Iacolino G, Ciaccio M. Vitamin D and the nervous system. Neurol Res 2019;41:827-35.


49. Garcia-Serna AM, Morales E. Neurodevelopmental effects of prenatal vitamin D in humans: systematic review and meta-analysis. Mol Psychiatry 2020;25:2468-81.



50. Zhou SH, Wang X, Fan MY, Li HL, Bian F, Huang T, et al. Influence of vitamin D deficiency on T cell subsets and related indices during spinal tuberculosis. Exp Ther Med 2018;16:718-22.



51. Karras SN, Wagner CL, Castracane VD. Understanding vitamin D metabolism in pregnancy: from physiology to pathophysiology and clinical outcomes. Metabolism 2018;86:112-23.


52. Mak A. The impact of vitamin D on the immunopathophysiology, disease activity, and extra-musculoskeletal manifestations of systemic lupus erythematosus. Int J Mol Sci 2018;19:2355.



53. Fahmy SF, Sabri NA, El Hamamsy MH, El Sawi M, Zaki OK. Vitamin D intake and sun exposure in autistic children. Int J Pharm Sci Res 2016;7:1043-9.
54. Basheer S, Natarajan A, van Amelsvoort T, Venkataswamy MM, Ravi V, Srinath S, et al. Vitamin D status of children with autism spectrum disorder: case-control study from India. Asian J Psychiatr 2017;30:200-1.


55. Cieslinska A, Kostyra E, Chwala B, Moszynska-Dumara M, Fiedorowicz E, Teodorowicz M, et al. Vitamin D receptor gene polymorphisms associated with childhood autism. Brain Sci 2017;7:115.



56. Desoky T, Hassan MH, Fayed HM, Sakhr HM. Biochemical assessments of thyroid profile, serum 25-hydroxycholecalciferol and cluster of differentiation 5 expression levels among children with autism. Neuropsychiatr Dis Treat 2017;13:2397-403.




57. Garipardic M, Dogan M, Bala KA, Mutluer T, Kaba S, Aslan O, et al. Association of attention deficit hyperactivity disorder and autism spectrum disorders with mean platelet volume and vitamin D. Med Sci Monit 2017;23:1378-84.



58. Altun H, Kurutas EB, Sahin N, Gungor O, Findikli E. The levels of vitamin D, vitamin D receptor, homocysteine and complex b vitamin in children with autism spectrum disorders. Clin Psychopharmacol Neurosci 2018;16:383-90.



59. Arastoo AA, Khojastehkia H, Rahimi Z, Khafaie MA, Hosseini SA, Mansouri MT, et al. Evaluation of serum 25-hydroxy vitamin D levels in children with autism spectrum disorder. Ital J Pediatr 2018;44:150.




60. Dong HY, Wang B, Li HH, Shan L, Jia FY. Correlation between serum 25-hydroxyvitamin D level and core symptoms of autism spectrum disorder in children. Zhonghua Er Ke Za Zhi 2017;55:916-9.

61. Guo M, Zhu J, Yang T, Lai X, Lei Y, Chen J, et al. Vitamin A and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutr Neurosci 2019;22:637-47.


62. Lopez-Vicente M, Sunyer J, Lertxundi N, Gonzalez L, Rodriguez-Dehli C, Espada Saenz-Torre M, et al. Maternal circulating vitamin D3 levels during pregnancy and behaviour across childhood. Sci Rep 2019;9:14792.




63. Chen J, Xin K, Wei J, Zhang K, Xiao H. Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J Psychosom Res 2016;89:98-101.


64. Vinkhuyzen AA, Eyles DW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, et al. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol Psychiatry 2018;23:240-6.




65. Wang T, Shan L, Du L, Feng J, Xu Z, Staal WG, et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry 2016;25:341-50.



66. Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Sconberg JL, Schmidt LC, et al. Selected vitamin D metabolic gene variants and risk for autism spectrum disorder in the CHARGE Study. Early Hum Dev 2015;91:483-9.



67. Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441:61-76.




68. Trifonova EA, Klimenko AI, Mustafin ZS, Lashin SA, Kochetov AV. The mTOR signaling pathway activity and vitamin D availability control the expression of most autism predisposition genes. Int J Mol Sci 2019;20:6332.



69. Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila) 2009;2:213-23.




70. Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 2011;68:467-76.



71. Marini F, Bartoccini E, Cascianelli G, Voccoli V, Baviglia MG, Magni MV, et al. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 2010;20:696-705.


72. Ali A, Cui X, Eyles D. Developmental vitamin D deficiency and autism: putative pathogenic mechanisms. J Steroid Biochem Mol Biol 2018;175:108-18.


73. Staal WG, de Krom M, de Jonge MV. Brief report: the dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in autism spectrum disorder (ASD). J Autism Dev Disord 2012;42:885-8.



74. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J 2014;28:2398-413.



75. Pertile RA, Cui X, Hammond L, Eyles DW. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J 2018;32:819-28.



76. Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry 2010;67:59-68.


77. Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: relation to autoimmunity. J Neuroinflammation 2012;9:201.




78. El-Sharkawy A, Malki A. Vitamin D signaling in inflammation and cancer: molecular mechanisms and therapeutic implications. Molecules 2020;25:3219.



79. Napolioni V, Ober-Reynolds B, Szelinger S, Corneveaux JJ, Pawlowski T, Ober-Reynolds S, et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J Neuroinflammation 2013;10:38.




80. Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, et al. Neonatal cytokine profiles associated with autism spectrum disorder. Biol Psychiatry 2017;81:442-51.



81. Mazahery H, Camargo CA, Conlon C, Beck KL, Kruger MC, von Hurst PR. Vitamin D and autism spectrum disorder: a literature review. Nutrients 2016;8:236.



82. Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA 2010;304:2389-96.



83. James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 2006;141B:947-56.



85. Fu L, Chen YH, Chen X, Xu S, Yu Z, Xu DX. Vitamin D deficiency impairs neurobehavioral development in male mice. Physiol Behav 2017;179:333-9.


86. Stubbs G, Henley K, Green J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses 2016;88:74-8.


87. Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ 2017;359:j5237.



88. Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011;128:e488-95.




89. Principi N, Esposito S. Vitamin D deficiency during pregnancy and autism spectrum disorders development. Front Psychiatry 2020;10:987.



90. Jia F, Wang B, Shan L, Xu Z, Staal WG, Du L. Core symptoms of autism improved after vitamin D supplementation. Pediatrics 2015;135:e196-8.



91. Azzam HME, Sayyah H, Youssef S, Lotfy H, Abdelhamid IA, Abd Elhamed HA, et al. Autism and vitamin D. Middle East Curr Psychiatry 2015;22:9-14.

92. Feng J, Shan L, Du L, Wang B, Li H, Wang W, et al. Clinical improvement following vitamin D3 supplementation in autism spectrum disorder. Nutr Neurosci 2017;20:284-90.


- TOOLS