|
 |
- Search
| Ann Child Neurol > Volume 32(1); 2024 > Article |
|
Abstract
Purpose
The incidence and mortality rates of arterial ischemic stroke (AIS) among pediatric patients have been frequently reported. While pediatric stroke can have multiple severe effects, its risk factors have not been methodically examined. This systematic review and meta-analysis were completed to summarize the existing evidence regarding risk factors for AIS in pediatric patients.
Methods
To gather relevant articles published in the past 15 years, searches were conducted of PubMed and Scopus. Multivariate odds ratios (ORs) and 95% confidence intervals (CIs) were analyzed using Review Manager 5.4.
Results
From the initial screening of 507 articles, five articles comprising a total of 1,423 participants were selected for qualitative analysis. Two of these additionally underwent quantitative analysis. Among the total participants, 1,108 children with AIS (77.9%) had arteriopathy as the underlying disease. Types of arteriopathy included moyamoya disease (24.28%), arterial dissection (23.29%), focal cerebral arteriopathy (16.16%), and vasculitis (14.71%). The meta-analysis revealed that being between 6 and 9 years of age (OR, 2.19; 95% CI, 1.76 to 2.73; P<0.00001) and having sickle cell disease (OR, 3.46; 95% CI, 2.38 to 5.05; P<0.00001) were associated with arteriopathy in pediatric AIS.
A stroke is defined as a sudden onset of focal or global neurological deficit attributed to cerebrovascular injury, such as an infarction or hemorrhage of the central nervous system, that persists for longer than 24 hours [1]. Stroke is the second leading cause of death worldwide [2]. Arterial ischemic stroke (AIS) is responsible for approximately 85% of all strokes, while the remaining cases are due to intracerebral hemorrhage [3].
Stroke can occur at any age. Despite its rarity in children, pediatric stroke is associated with high morbidity and mortality rates, which may stem from delayed diagnosis and a lack of awareness of contributing risk factors [4]. Pediatric stroke is classified into two primary categories based on age. Perinatal stroke is categorized as occurring in neonates, from birth to 28 days of life. In contrast, stroke occurring in children from 29 days to 18 years old is classified as childhood stroke [5-7].
AIS in pediatric patients is a multifactorial disease, influenced by a broad array of risk factors. From 1992 to 2001, the incidence of ischemic stroke among 1,129 pediatric patients was reported to be 1.72 per 100,000 persons annually for childhood stroke and 10.2 per 100,000 live births for perinatal stroke [8]. Separate research published in 2019 reported an incidence of perinatal stroke at 1 per 3,500 live births, along with an incidence of childhood stroke between 1 and 2 per 100,000 persons annually [6]. The in-hospital mortality rate of stroke in children has been reported to account for 2.6% of all pediatric stroke cases [9].
The incidence of AIS in pediatric populations has been widely reported, and this condition can lead to numerous severe consequences. However, its risk factors have not been systematically investigated. Early identification and immediate mitigation of these factors can help minimize morbidity and mortality. This systematic review and meta-analysis were conducted to consolidate the existing evidence on the risk factors for AIS in pediatric populations.
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines. The design and protocol were registered with PROSPERO (registration number CRD42023454039) on August 24, 2023.
Included articles were required to examine the associations of sickle cell disease and other risk factors with AIS in newborns through children up to 18 years old. Additionally, eligible articles had to be observational human studies with retrospective or prospective cohort designs that were published within the prior 15 years and had a minimum sample size of 10. To be selected for meta-analysis, articles needed to provide odds ratio (OR) and 95% confidence interval (CI) values. Case reports, literature reviews, systematic reviews, meta-analyses, animal studies, and articles without full text available were excluded.
Articles were systematically searched in PubMed and Scopus, using a combination of free-text searching and keyword probing. The search terms employed were as follows: ("risk factor" OR "risk factors" OR "predictor" OR "predictors") AND ("stroke" OR "ischemic stroke" OR "arterial ischemic stroke") AND ("pediatric" OR "neonates" OR "perinatal" OR "childhood" OR "children"). The reference lists from the resulting articles were manually reviewed to identify additional relevant studies.
Four authors (CPT, LW, EHT, and AMG) manually screened the titles and abstracts based on the eligibility criteria. The full texts of the selected articles underwent a second round of evaluation. Additionally, the Newcastle-Ottawa scale (NOS) instrument was employed to assess the articles. In instances involving disagreement, the fifth author (NMDP) was consulted to facilitate a consensus.
The data from the chosen articles were extracted by four authors (CPT, LW, EHT, and AMG) as follows: first author, publication date, country, population, sample size, and outcomes. The statistical analysis for the meta-analysis was conducted using Review Manager (RevMan) version 5.4 (Stata Corp., College Station, TX, USA).
A total of 507 articles were identified through searches of PubMed and Scopus. The initial screening led to the exclusion of 479 articles, which included 332 duplicates, 68 articles without full text, 57 articles in which the variable was not ischemic stroke, and 22 articles that were neither case-control nor cohort studies. The second screening involved a full-text review of the remaining 28 articles to determine their eligibility, resulting in the exclusion of 23 articles. Ultimately, five articles were deemed suitable for the systematic review, and two were included in the meta-analysis (Fig. 1).
The quality of each article was evaluated using the NOS instrument, as shown in Table 1 [10-14]. Articles were categorized based on their total scores: a score of 7 to 10 indicated high quality, a score of 4 to 6 suggested a low risk of bias, and a score of 1 to 3 denoted a high risk of bias. Among the articles assessed, two received a total score of 8 out of 10, one scored 7, one scored 6, and one scored 5. Thus, three articles were deemed high quality, while two were considered to have a low risk of bias.
The articles included in this review were published between 2009 and 2020 and encompass two case-control studies and three cohort studies, as detailed in Table 2. These studies were conducted across various regions, including North America, South America, Europe, Australia, Asia, the United States, Taiwan, Germany, and Iran, and involved a total of 1,423 participants. The participant population in three of the studies consisted of children ranging in age from 28-30 days to 18-19 years, while the remaining two studies focused on neonates and children under the age of 18. The diagnostic criteria for AIS were derived as follows: two articles utilized clinical neurological deficit and radiological data from the International Pediatric Stroke Study; one article employed the International Classification of Diseases, Ninth Revision coding system; another article relied on diagnoses from pediatric neurologists and the German Paediatric Surveillance Unit; and one article based its assessments on brain magnetic resonance imaging.
In the review of five studies involving 1,423 pediatric patients with AIS, the underlying diseases were found to be arteriopathy (77.9%), cardiac disorder (4.36%), hemato-oncological disorder (2.95%), infection (2.81%), and prothrombotic state (2.18%). Other conditions were also identified, including severe renal disorder (1.97%), sequelae of previous brain damage (0.84%), acute systemic condition (0.77%), chronic head and neck disorder (0.63%), anoxic brain injury (0.49%), prematurity (0.49%), metabolic disorder (0.49%), non-abusive head trauma (0.42%), retardation of physiological development or nutritional problems (0.35%), volume depletion (0.35%), abusive head trauma or shaken baby syndrome (0.35%), central nervous system anomaly (0.28%), chronic systemic condition (0.28%), mitochondrial disease (0.14%), other traumatic injuries (0.14%), chromosomal or congenital anomaly (0.07%), acute head and neck disorder (0.07%), and other unspecified conditions (2.53%).
In a study of 1,108 pediatric patients with AIS, the types of arteriopathy identified were moyamoya (24.28%), arterial dissection (23.29%), focal cerebral arteriopathy (16.16%), and vasculitis (14.71%). Other arteriopathies detected included sickle cell disease (1.9%), post-varicella angiopathy (1.71%), cerebral aortic aneurysm (1.62%), cerebral artery occlusion (0.54%), neurofibromatosis type I-related vascular dysplasia (0.36%), systemic lupus erythematosus (0.18%), arteriovenous malformation (0.18%), other peripheral vascular anomalies (0.18%), Ehlers-Danlos syndrome (0.09%), cerebral atherosclerosis (0.09%), carotid artery stenosis (0.09%), vertebral artery occlusion (0.09%), and other types of arteriopathy not specified (14.62%).
Two articles with the same outcome variables were combined for meta-analysis. This meta-analysis examined the associations between an age of 6 to 9 years, male sex, sickle cell disease, and cardiac disease in relation to arteriopathy in pediatric AIS. As depicted in Fig. 2, an age between 6 and 9 years (OR, 2.19; 95% CI, 1.76 to 2.73; P<0.00001) and sickle cell disease (OR, 3.46; 95% CI, 2.38 to 5.05; P<0.00001) were significantly associated with AIS in pediatric patients. Arteriopathy in pediatric AIS was found to have a significant negative association with cardiac disease (OR, 0.29; 95% CI, 0.23 to 0.35; P<0.00001); however, no significant association was found with male sex (OR, 0.98; 95% CI, 0.83 to 1.15; P=0.77).
Pediatric AIS is a multifactorial disease with a variety of underlying causes, some of which may have been previously unrecognized as contributing factors. Rather than focusing solely on therapeutic interventions for the disease, identifying these risk factors can serve as an effective strategy to decrease its incidence. Early recognition of risk factors presents opportunities to reduce both the mortality and morbidity rates associated with pediatric AIS.
The qualitative analysis presented in Table 2 reveals a variety of underlying diseases associated with pediatric AIS. Of these, arteriopathy emerged as the most frequently occurring disease across the five included studies. More specifically, moyamoya disease was identified as the most common type of arteriopathy in pediatric AIS. Moyamoya disease is a chronic cerebrovascular condition of unknown origin, characterized by enduring stenosis of the intracranial blood vessels. It most commonly affects the terminal portion of the internal carotid and middle cerebral arteries [15]. This occlusion of the large intracranial artery contributes to hemodynamic disturbances, resulting in hypoperfusion that can induce hypoxic injuries in brain cells [16].
Artery dissection is the second most common type of arteriopathy. This condition arises when a tear forms within the inner lining of the arterial wall. The compromised structure of the inner arterial vessel allows blood flow to reach these torn areas, which may lead to clot formation. These unstable clots can disrupt blood circulation, including that of the brain, thereby interfering with cerebral perfusion and potentially resulting in ischemic stroke [7,17]. Notably, several medical conditions (such as Ehlers-Danlos syndrome, vasculitis, and head or neck trauma) that have been identified as underlying diseases and other types of arteriopathy in pediatric AIS have also been associated with arterial dissection [6,9,18-20].
The quantitative analysis presented in Fig. 2 indicates an association between sickle cell disease and AIS in pediatric patients. Sickle cell disease is a hereditary disorder resulting from a defect in the hemoglobin gene on chromosome 11, specifically the beta-globin component [21]. This single base-pair point mutation disrupts the function of hemoglobin, a molecule within erythrocytes responsible for delivering oxygen to cells [22]. In sickle cell anemia, the most common form of sickle cell disease, severe anemia can lead to a reduction in oxygen perfusion to the brain [23]. Vasculopathy is also predominantly observed in patients with sickle cell disease [24]. Furthermore, sickle cell disease is associated with an elevated risk of thrombophilia, which can disrupt the delivery of oxygen to brain tissue [25].
Children between the ages of 6 and 9 years have been found to have a heightened risk of AIS compared to other age groups [10,11]. This finding may be attributed to the fact that children aged 6 years and older tend to develop more advanced motor skills, which carry a higher risk of trauma compared to the motor skills of younger children. These skills include activities such as riding a two-wheeled bicycle and other tasks that require coordination with larger muscles. Furthermore, children aged 9 years and under typically have not yet developed stable self-control [26,27]. The ability to control and regulate impulsivity is age-dependent and aligns with the maturation of the prefrontal cortex, the center for impulse control. This maturation generally occurs in children aged 10 years and above [28]. Consequently, these factors contribute to children aged 6 to 9 years exhibiting an elevated risk of traumatic brain injury, which is a risk factor for AIS in pediatric populations [12,29].
A negative correlation was identified between a history of cardiac disease and arteriopathy in pediatric AIS, based on the combined OR results of two included studies. Children with cardiac disease were less likely to experience AIS than children without cardiac disease. This is likely due to incomplete vascular imaging in both cardiac and non-cardiac patients, as detailed in the included studies [10,11]. However, these results should be interpreted with caution due to the potential use of antiplatelet agents in patients with cardiac disease. Research indicates that antiplatelet agents can reduce the risk of AIS in pediatric patients [30]. In contrast, cardiac disorder has been found to increase the risk of pediatric AIS [12-14,31-34]. Future research on the relationship between cardiac disease and AIS in pediatric patients should be more specific, focusing on diagnostic investigation, medication history, and other potentially influential factors.
In alignment with previous studies, the findings did not reveal a significant association between male sex and pediatric AIS [12,33,35]. However, other research has identified a significant male predominance in the incidence of AIS in pediatric populations [7,13,36,37]. Further studies are needed to explore the relationship between sex and pediatric AIS, including potential underlying mechanisms.
This study provides evidence of risk factors for AIS in pediatric populations, based on both qualitative analysis and quantitative analysis with a high statistical conclusion value. However, this study had several limitations due to confounding factors. Notably, the analysis included a small number of studies, which resulted from the limited number of updated articles discussing pediatric AIS over the past decade. Future research on pediatric AIS should be conducted with a larger population. Additionally, further studies should be performed to explore the association between existing risk factors and AIS in pediatric patients to uncover the underlying mechanism.
In conclusion, arteriopathy is the most common risk factor for AIS in pediatric patients. The age range of 6 to 9 years, along with the presence of sickle cell disease, are the primary risk factors for arteriopathy in pediatric AIS.
Notes
Author contribution
Conceptualization: CPT and LW. Data curation: CPT, LW, EHT, and AMG. Formal analysis: CPT, LW, EHT, AMG, and NMDP. Methodology: CPT and LW. Writing-original draft: CPT. Writing-review & editing: CPT, LW, EHT, AMG, and NMDP.
Acknowledgments
The authors express their gratitude to the Faculty of Medicine at Widya Mandala Catholic University Surabaya in Surabaya, Indonesia, as well as the Department of Neurology at the Faculty of Medicine, Udayana University in Bali, Indonesia, for their support.
Fig. 2.
Meta-analysis of (A) 6 to 9 years of age, (B) male gender, (C) sickle cell disease, and (D) cardiac disease using odds ratio in pediatric arterial ischemic stroke with and without arteriopathy. M-H, Mantel-Haenszel; CI, confidence interval.
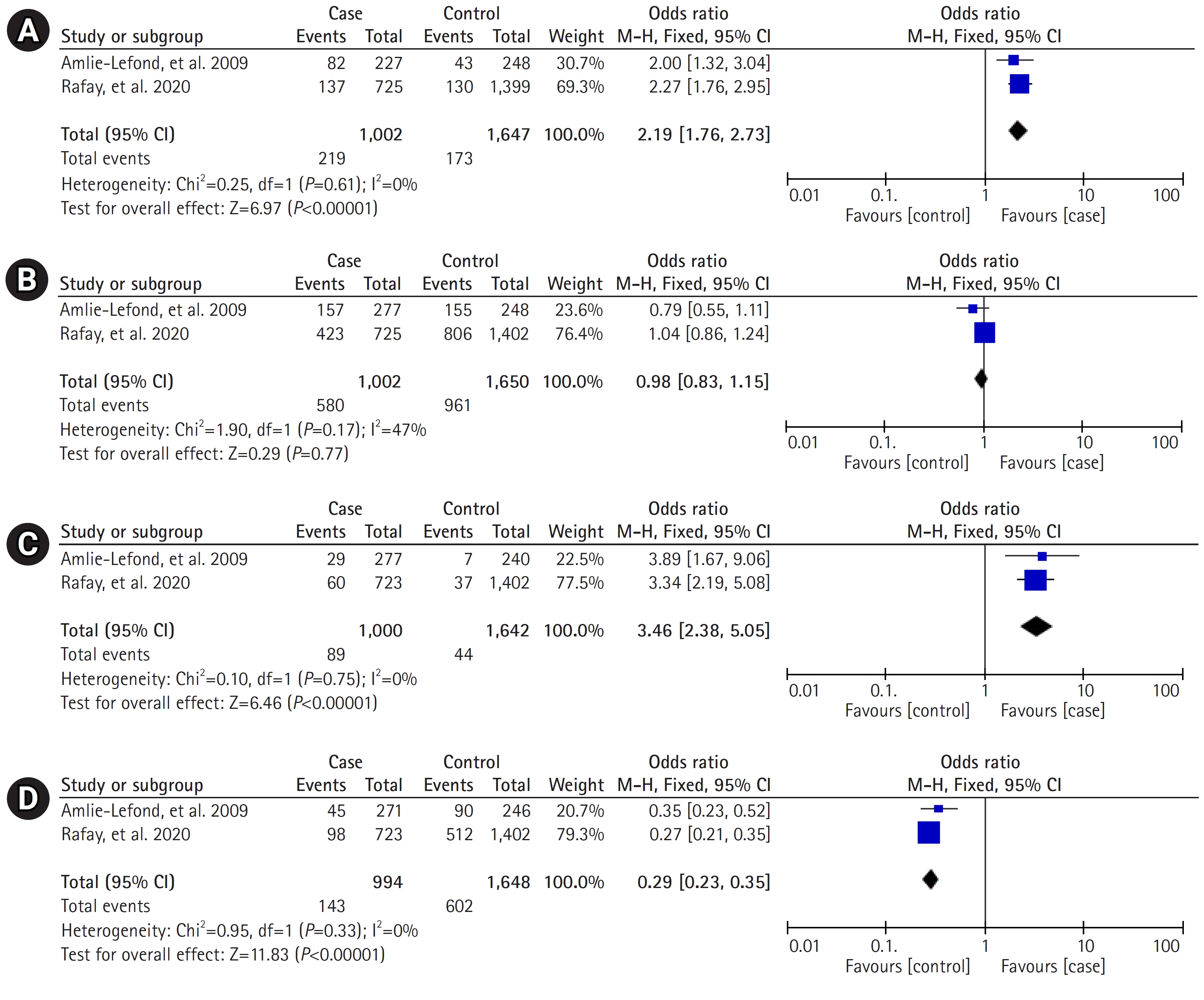
Table 1.
Newcastle-Ottawa scale
| Study | Study design | Score | Total score | ||
|---|---|---|---|---|---|
| Selection | Comparability | Exposure | |||
| Amlie-Lefond et al. (2009) [10] | Case-control | 3 | 2 | 2 | 7 |
| Rafay et al. (2020) [11] | Case-control | 4 | 2 | 2 | 8 |
| Chiang et al. (2018) [12] | Cohort | 4 | 1 | 3 | 8 |
| Gerstl et al. (2018) [13] | Cohort | 2 | 2 | 2 | 6 |
| Ghofrani et al. (2018) [14] | Cohort | 3 | 0 | 2 | 5 |
Table 2.
Summary of study characteristics
| Study | Country or region | Population | Sample size (n) | AIS-related factor | Type of arteriopathy | Arteriopathy-related factor |
|---|---|---|---|---|---|---|
| Amlie-Lefond et al. (2009) [10] | United States, North America, South America, Europe, Australia, Asia | Children 29 days to 19 years old with AIS, confirmed by clinical and radiographic data | 278 | Arteriopathy | Focal cerebral arteriopathy (n=69) | Age 6-9 years (OR, 2.00; 95% CI, 1.32-3.04; P=0.001) |
| Moyamoya (n=61) | Male sex (OR, 0.79; 95% CI, 0.55-1.11; P=0.175) | |||||
| Arterial dissection (n=56) | Sickle cell disease (OR, 3.89; 95% CI, 1.67-9.06; P=0.013) | |||||
| Vasculitis (n=33) | Cardiac disease (OR, 0.35; 95% CI, 0.23-0.52; P<0.0001) | |||||
| Sickle cell disease (n=21) | ||||||
| Post-varicella angiopathy (n=19) | ||||||
| Unspecified vasculopathy (n=9) | ||||||
| Other (n=19) | ||||||
| Rafay et al. (2020) [11] | North America, South America, Europe, Australia, Asia | Children 1 month to 18 years old with AIS, presenting with acute neurological deficit and confirmed by radiologic pattern of arterial ischemia | 725 | Arteriopathy | Arterial dissection (n=193) | Age 6-9 years (OR, 2.27; 95% CI, 1.76-2.95; P=0.029) |
| Moyamoya (n=177) | Male sex (OR, 1.04; 95% CI, 0.86-1.24) | |||||
| Vasculitis (n=111) | Sickle cell disease (OR, 3.34; 95% CI, 2.19-5.08; P=0.007) | |||||
| Focal cerebral arteriopathy (n=110) | Cardiac disease (OR, 0.27; 95% CI, 0.21-0.35) | |||||
| Other (n=134) | ||||||
| Chiang et al. (2018) [12] | Taiwan | Children under 18 years old registered as having AIS in ICD-9 coding data | 247 | Arteriopathy (n=73) | Moyamoya (n=20) | - |
| Infection (n=38) | Vasculitis (n=15) | |||||
| Cardiac disorder (n=25) | NF-1-related vascular dysplasia (n=4) | |||||
| Hemato-oncological disorder (n=33) | Cerebral aortic aneurysm (n=18) | |||||
| Severe renal disorder (n=28) | AV malformation (n=2) | |||||
| Anoxic brain injury (n=7) | Ehlers-Danlos syndrome (n=1) | |||||
| Prematurity (n=7) | Cerebral atherosclerosis (n=1) | |||||
| Non-abusive head trauma (n=6) | Carotid artery stenosis (n=1) | |||||
| Metabolic disorder (n=6) | Vertebral artery occlusion (n=1) | |||||
| Retardation of physiological development or nutritional problems (n=5) | Cerebral artery occlusion (n=6) | |||||
| Volume depletion (n=5) | Other unmentioned cerebral vascular anomaly (n=4) | |||||
| Abusive head trauma or shaken baby (n=5) | ||||||
| CNS anomaly (n=4) | ||||||
| Mitochondrial disease (n=2) | ||||||
| Other traumatic injuries (n=2) | ||||||
| Chromosome or congenital anomaly (n=1) | ||||||
| Other sequelae of previous brain damage (n=12) | ||||||
| Gerstl et al. (2018) [13] | Germany | Children from 28 days to 18 years old with AIS, diagnosed by a pediatric neurologist and identified using ESPED | 111 | Cardiac disorder (n=24) | Primary CNS vasculitis (n=1) | - |
| Prothrombotic state (n=28) | Para-/post-infectious vasculitis (n=4) | |||||
| Arteriopathy (n=21) | Arterial dissection (n=9) | |||||
| Acute systemic condition (n=9) | Moyamoya (n=4) | |||||
| Hemato-oncological disorder (n=9) | SLE (n=2) | |||||
| Chronic head and neck disorder (n=9) | Other (n=1) | |||||
| Metabolic disorder (n=1) | ||||||
| Other (n=10) | ||||||
| Ghofrani et al. (2018) [14] | Iran | Pediatric patients 0-18 years old with AIS, confirmed by brain MRI | 62 | Cardiac disorder (n=13) | Moyamoya (n=7) | - |
| Arteriopathy (n=11) | Other (n=4) | |||||
| Infection (n=2) | ||||||
| Acute systemic condition (n=2) | ||||||
| Chronic systemic condition (n=4) | ||||||
| Prothrombotic state (n=3) | ||||||
| Acute head and neck disorder (n=1) | ||||||
| Other (n=26) |
AIS, arterial ischemic stroke; OR, odds ratio; CI, confidence interval; ICD-9, International Classification of Diseases, Ninth Revision; NF-1, neurofibromatosis type I; AV, arteriovenous; ESPED, German Paediatric Surveillance Unit; CNS, central nervous system; SLE, systemic lupus erythematosus; MRI, magnetic resonance imaging.
References
1. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89.


2. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke 2022;17:18-29.



3. GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439-58.


4. Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Diagnostic delays in paediatric stroke. J Neurol Neurosurg Psychiatry 2015;86:917-21.


5. Malone LA, Felling RJ. Pediatric stroke: unique implications of the immature brain on injury and recovery. Pediatr Neurol 2020;102:3-9.



6. Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke 2019;50:e51-96.


7. deVeber GA, Kirton A, Booth FA, Yager JY, Wirrell EC, Wood E, et al. Epidemiology and outcomes of arterial ischemic stroke in children: the Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol 2017;69:58-70.


8. Beslow LA, Dowling MM, Hassanein SM, Lynch JK, Zafeiriou D, Sun LR, et al. Mortality after pediatric arterial ischemic stroke. Pediatrics 2018;141:e20174146.



9. Felling RJ, Sun LR, Maxwell EC, Goldenberg N, Bernard T. Pediatric arterial ischemic stroke: epidemiology, risk factors, and management. Blood Cells Mol Dis 2017;67:23-33.


10. Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 2009;119:1417-23.



11. Rafay MF, Shapiro KA, Surmava AM, deVeber GA, Kirton A, Fullerton HJ, et al. Spectrum of cerebral arteriopathies in children with arterial ischemic stroke. Neurology 2020;94:e2479-90.



12. Chiang KL, Cheng CY. Epidemiology, risk factors and characteristics of pediatric stroke: a nationwide population-based study. QJM 2018;111:445-54.


13. Gerstl L, Weinberger R, von Kries R, Heinen F, Schroeder AS, Bonfert MV, et al. Risk factors in childhood arterial ischaemic stroke: findings from a population-based study in Germany. Eur J Paediatr Neurol 2018;22:380-6.


14. Ghofrani M, Tonekaboni H, Karimzadeh P, Nasiri J, Pirzadeh Z, Ghazzavi M, et al. Risk factors of pediatric arterial ischemic stroke; a regional survey. Int J Prev Med 2018;9:69.



15. Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: treatment and outcomes. J Stroke 2016;18:21-30.




16. Fan AP, Khalighi MM, Guo J, Ishii Y, Rosenberg J, Wardak M, et al. Identifying hypoperfusion in moyamoya disease with arterial spin labeling and an [15O]-water positron emission tomography/magnetic resonance imaging normative database. Stroke 2019;50:373-80.



17. Brinjikji W, Nogueira RG, Kvamme P, Layton KF, Delgado Almandoz JE, Hanel RA, et al. Association between clot composition and stroke origin in mechanical thrombectomy patients: analysis of the Stroke Thromboembolism Registry of Imaging and Pathology. J Neurointerv Surg 2021;13:594-8.



18. Hollist M, Au K, Morgan L, Shetty PA, Rane R, Hollist A, et al. Pediatric stroke: overview and recent updates. Aging Dis 2021;12:1043-55.



19. Nash M, Rafay MF. Craniocervical arterial dissection in children: pathophysiology and management. Pediatr Neurol 2019;95:9-18.


20. Kim ST, Brinjikji W, Lanzino G, Kallmes DF. Neurovascular manifestations of connective-tissue diseases: a review. Interv Neuroradiol 2016;22:624-37.




21. Hardouin G, Magrin E, Corsia A, Cavazzana M, Miccio A, Semeraro M. Sickle cell disease: from genetics to curative approaches. Annu Rev Genomics Hum Genet 2023;24:255-75.


22. Thein SL. Genetic basis and genetic modifiers of β-thalassemia and sickle cell disease. Adv Exp Med Biol 2017;1013:27-57.


23. Pedrosa AM, Lemes RP. Gene expression of HIF-1α and VEGF in response to hypoxia in sickle cell anaemia: influence of hydroxycarbamide. Br J Haematol 2020;190:e39-42.



24. Nader E, Conran N, Romana M, Connes P. Vasculopathy in sickle cell disease: from red blood cell sickling to vascular dysfunction. Compr Physiol 2021;11:1785-803.



25. Wun T, Brunson A. Sickle cell disease: an inherited thrombophilia. Hematology Am Soc Hematol Educ Program 2016;2016:640-7.




26. Centers for Disease Control and Prevention (CDC). Percent distributions of TBI-related deaths by age group and injury mechanism: United States, 2006-2010 [Internet] Atlanta: CDC; 2016 [cited 2023 Nov 14]. Available from: https://www.cdc.gov/traumaticbraininjury/data/dist_death.html
27. Chen HY, Meng LF, Yu Y, Chen CC, Hung LY, Lin SC, et al. Developmental traits of impulse control behavior in school children under controlled attention, motor function, and perception. Children (Basel) 2021;8:922.



28. Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu Rev Neurosci 2015;38:151-70.



29. Kowalski RG, Haarbauer-Krupa JK, Bell JM, Corrigan JD, Hammond FM, Torbey MT, et al. Acute ischemic stroke after moderate to severe traumatic brain injury: incidence and impact on outcome. Stroke 2017;48:1802-9.



30. Soman T, Rafay MF, Hune S, Allen A, MacGregor D, deVeber G. The risks and safety of clopidogrel in pediatric arterial ischemic stroke. Stroke 2006;37:1120-2.


31. Chung MG, Guilliams KP, Wilson JL, Beslow LA, Dowling MM, Friedman NR, et al. Arterial ischemic stroke secondary to cardiac disease in neonates and children. Pediatr Neurol 2019;100:35-41.



32. Shiva S, Barzegar M, Rashidzadeh M. Epidemiology and outcomes of arterial ischemic stroke in children admitted to Tabriz Children’s Hospital, Tabriz, Iran during (2014-2019). Int J Pediatr 2021;9:13887-94.
33. Mallick AA, Ganesan V, Kirkham FJ, Fallon P, Hedderly T, McShane T, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol 2014;13:35-43.


34. Fox CK, Sidney S, Fullerton HJ. Community-based case-control study of childhood stroke risk associated with congenital heart disease. Stroke 2015;46:336-40.



35. Sultan S, Schupf N, Dowling M, DeVeber G, Kirton A, Elkind MS, et al. Predictors of cholesterol and lipoprotein(a) testing in children with arterial ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:2405-13.



- TOOLS