Sphenoid Sinus Mucocele Causing Orbital Apex Syndrome, Methicillin-Susceptible Staphylococcus aureus Bacteremia, and Hypopituitarism
Article information
Sphenoid sinus mucocele (SSM) is a rare disease, accounting for only 1% to 2% of all paranasal sinus mucoceles [1]. Typically, paranasal sinus mucoceles develop after radiotherapy or in patients with cystic fibrosis; however, some studies have reported SSM with no specific underlying cause other than chronic rhinitis [2]. Although SSM is benign, it tends to expand, leading to severe complications such as optic nerve palsy, ocular motor nerve palsy, and hypopituitarism [3]. In the event of these complications, urgent decompression is advised; however, complete recovery of ocular symptoms is not guaranteed even with immediate treatment [4].
In this report, we present the case of an adolescent boy diagnosed with SSM, orbital apex syndrome, and hypopituitarism who experienced serious vision loss despite active treatment. This case was reviewed and approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2303-014-125). Informed consent was obtained from the carer. The patient’s medical records and other data were anonymized to ensure confidentiality. A 17-year-old boy presented with a sudden onset of blindness and ptosis in his left eye, which had persisted for 1 day, as well as a headache in the left parietotemporal area that had lasted for 3 days. He had been diagnosed with mild intellectual disability and had experienced several febrile convulsions between the ages of 2 and 5 years. Brain magnetic resonance imaging (MRI) and electroencephalography performed at those times revealed normal results. The patient was able to describe his physical condition independently. Apart from a history of frequent rhinitis and sinusitis for several years, he was generally healthy.
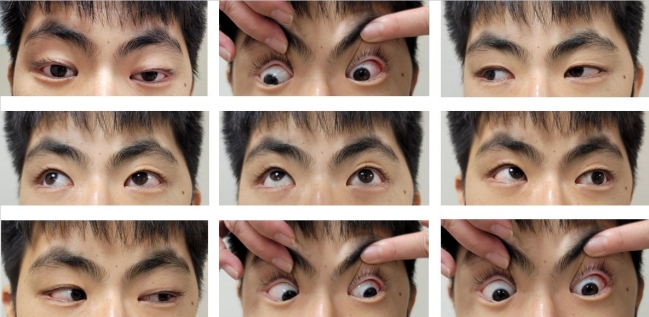
The patient’s vital signs were as follows: blood pressure, 110/70 mm Hg; pulse rate, 72 beats per minute; and body temperature, 39°C, which was the first recorded fever. Despite experiencing a frontal headache, he exhibited neither signs of meningeal irritation nor tenderness or warmth on his forehead upon examination. A cranial nerve examination revealed severely decreased visual acuity in his left eye, with no light perception (NLP) and multiple ocular motor cranial nerve palsies, including of the third and sixth nerves (Fig. 1). The visual acuity of his right eye was 0.63 with correction, extraocular movements were intact, and a visual field exam of his right eye yielded normal findings. Laboratory tests revealed an increased white blood cell count (16,470/μL [normal range, 4,500 to 11,000]), elevated C-reactive protein level (5.07 mg/dL [normal range, <0.5]), and decreased pituitary hormone levels: prolactin, 1.67 ng/mL (normal range, 3 to 18.8); adrenocorticotropic hormone, 6.28 pg/mL (normal range, 10 to 60); cortisol, 1.31 μg/dL (normal range, 5 to 25); and testosterone, 0.12 ng/mL (normal range, 2.67 to 10.12). An ophthalmic examination showed a normal fundus and intraocular pressure. Cerebrospinal fluid analysis revealed no specific findings. Brain MRI confirmed an SSM affecting the bilateral cavernous sinuses along with inflammation of the surrounding tissues (Fig. 2A and B). We administered 2 g of ceftriaxone intravenously (IV) every 12 hours as an empirical antibiotic before removal surgery.
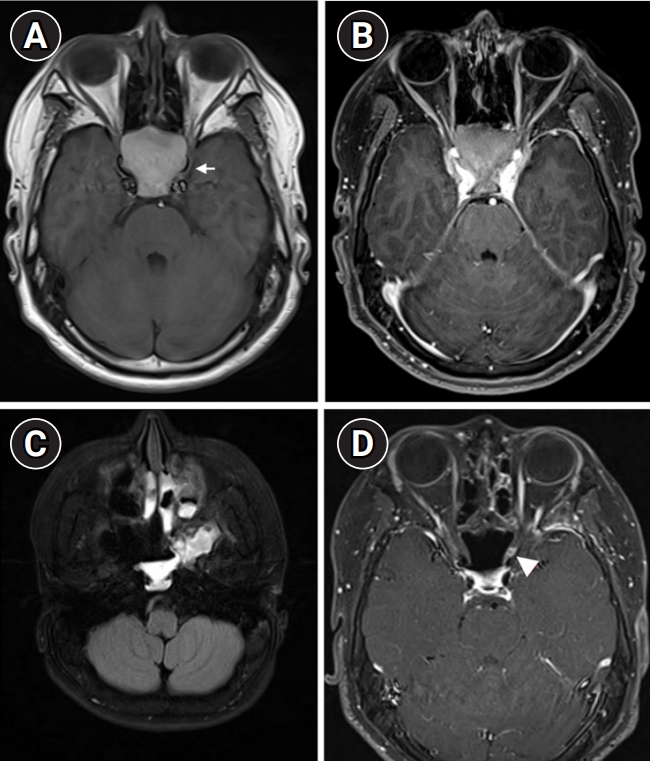
(A, B) Magnetic resonance imaging (MRI) on the day of admission. (A) Space-occupying lesions exhibiting T1 hyperintensities with expansion of bony structures (arrow). (B) Mass effect on the bilateral cavernous sinuses. (C, D) MRI on postoperative day 13. (C) Indeterminate viscous fluid present in the left sphenoid sinus. (D) Focal enhancement of the left optic nerve (arrowhead).
On the second day of admission, gram-positive cocci were isolated from the initial blood culture, and the C-reactive protein level increased to 21.25 mg/dL. We administered 500 mg of IV vancomycin every 6 hours for methicillin-resistant Staphylococcus aureus coverage. Preoperative IV dexamethasone (5 mg) was administered due to its known effect of reducing blood loss and improving the quality of the surgical field. Otolaryngologists performed endoscopic sinus surgery, and thick mucopurulent discharge was drained from both sphenoid sinuses. On the third day after admission, methicillin-susceptible Staphylococcus aureus (MSSA) was identified in both the pus from the sphenoid sinus and the initial blood culture. After diagnosing MSSA bacteremia originating from the mucocele, we changed the antibiotic regimen from ceftriaxone and vancomycin to ampicillin/sulbactam (3 g IV every 6 hours). On the 13th day after surgery, we observed a nearly complete recovery of ocular movements and resolution of sepsis on follow-up blood cultures; however, the patient’s visual acuity remained NLP. Postoperative MRI revealed probable left optic neuritis, slight improvement of previous cellulitis of the left masticator space, and remaining pus-like discharge in both sphenoid sinuses (Fig. 2C and D). Pulse steroid therapy (1 g/day IV for 5 days) was administered. After pulse therapy, oral cephalexin (500 mg every 6 hours) was continued for 40 days.
Following steroid pulse therapy, the patient's visual acuity remained NLP. His pituitary hormone levels normalized, with the exception of his testosterone levels. Even at 7 months postoperatively, the testosterone levels remained below the reference range, and a gonadotropin-releasing hormone stimulation test confirmed the presence of hypogonadotropic hypogonadism. The patient is currently receiving testosterone replacement therapy.
Orbital apex syndrome refers to a collection of symptoms resulting from the involvement of the orbital apex, which contains ocular motor nerves. Several studies have described orbital apex syndrome caused by SSM. A systematic review of visual prognosis in optic neuropathy due to SSM revealed varying outcomes regarding visual acuity, ranging from complete blindness to partial recovery. This contrasts with the resolution of diplopia and oculomotor nerve palsies. The review also indicated that the time to surgical removal or the use of adjunctive steroids may not significantly influence improvement in visual recovery [5]. The present patient underwent timely surgical decompression following the onset of symptoms and received steroids both preoperatively and postoperatively. However, his visual acuity remained NLP, which is consistent with the findings of the previous study.
Numerous studies have examined the use of postoperative antibiotics; however, the microbiological results of operative pus specimens from the surgical site have either not been described or have constituted negative findings [6,7]. In our case, the patient presented with fever and laboratory findings consistent with a bacterial infection, suggesting that a delay in diagnosis or antibiotic administration could have led to the spread of the infected mucocele to the meninges or even the adjacent cerebral tissues. To the best of our knowledge, SSM complicated by bacterial meningitis has not been reported previously, although many studies have identified meningitis as a potential complication of sinusitis. We recommend that the evaluation of patients with symptomatic SSM should include an assessment for concurrent systemic or central nervous system infections.
The decline in pituitary function may not always correlate well with the severity of visual symptoms. In previous case reports, a 10-year-old girl with bilateral visual loss due to SSM had normal pituitary gland hormone levels [8], while a 64-year-old SSM patient with hypopituitarism exhibited no visual symptoms [9]. In a case report of a patient with hypopituitarism and left-sided third and sixth nerve palsies, the cranial nerve palsies improved after decompensation, although the patient still required thyroxine [10]. Our patient required testosterone replacement.
SSM often presents with vague symptoms, necessitating thorough neurological examinations and timely imaging studies. Upon noticing decompensation, physicians must evaluate potential complications; this requires a multidisciplinary, long-term follow-up approach involving assessments of pituitary hormone levels, fundoscopic examinations, and additional imaging studies.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: YMK. Data curation: SEP. Project administration: YJL. Visualization: SEP. Writing-original draft: SEP. Writing-review & editing: SEP, YJL, YHJ, SHC, and YMK.