The Diagnostic Utility of Short-Term Video Electroencephalography at a Tertiary Care Center in North India: A Retrospective Study
Article information
Abstract
Purpose
This study investigated the diagnostic utility of short-term video electroencephalography (EEG) recordings at a tertiary care center in North India.
Methods
A retrospective analysis was conducted of 30 minutes of video EEG recordings done between January 2021 and January 2022 in children between 1 and 10 years of age. Demographic and clinical data were collected from the EEG register. Age, sex, the clinical diagnosis, the number of anti-seizure medicines (ASMs), the activation procedures used, and EEG abnormalities were recorded.
Results
Data from 100 children (male-to-female ratio, 1.9:1) were analysed. The mean age was 5.39±2.02 years. The indications for EEG recordings were epileptic disorders, neurodevelopmental disorders, paroxysmal non-epileptic events, and miscellaneous in 66%, 18%, 9%, and 7% of children, respectively. EEG abnormalities were seen in 50 children (50%) and about 45% of children were on two or more ASMs. EEG abnormalities in sleep were seen in 35 of 66 (53%) children, whereas abnormalities were observed on awake recordings with activation procedures (hyperventilation and photic) in 23 of 34 (68%) children; this difference was not statistically significant (P=0.16)
Conclusion
EEG abnormalities were significantly more common in children taking multiple ASMs; however, there was no statistically significant difference in the EEG yield between asleep and awake records with activation procedures. A better selection of patients for routine EEG, through an assessment of their clinical history and comorbidities, is warranted to increase its diagnostic yield.
Introduction
Video electroencephalographic (VEEG) recordings are an important diagnostic tool in neurology practise for epilepsy diagnosis and management [1]. VEEG is considered a gold standard in the diagnosis of pseudo-seizures or psychogenic non-epileptic seizures [2] Prolonged VEEG monitoring requires well-trained staff and infrastructure, which is difficult in a resource-limited settings [3]. Short-term VEEG recordings are economical and cost-effective since they do not require hospital admission and are therefore less costly than overnight electroencephalography (EEG) recordings. Studies have shown that even short-term recordings help to distinguish between epileptic and non-epileptic events and can help classify different seizure types [4]. However, only a few studies from North India have investigated the demographic profile and the role of short-term, outpatient VEEG in epilepsy and other disorders in the pediatric population [5]. The present study was undertaken to investigate the role of short-term VEEG in detecting the nature of abnormal events and to determine the utility of VEEG in confirming or classifying the referring diagnosis.
Materials and Methods
The study design was retrospective and hospital-based, involving an analysis of 30 minutes of short-term VEEG recordings done between January 2021 and January 2022 at a tertiary care center in North India. The short-term VEEGs were all done at the Department of Pediatric Neurology of this tertiary care center. Demographic and clinical data were collected from the EEG register. Children between 1 and 10 years of age were included, and their age, sex, clinical diagnosis, number of anti-seizure medicines (ASMs), activation procedures used, and EEG abnormalities were recorded. Activation procedures were done as appropriate. Children were sleep-deprived for all asleep records. Photic activation was done for all recordings (both asleep and awake). Eye opening and closure were performed by cooperative children, and passive eye closure was done for young children by having a technician cover their eyes. Hyperventilation was performed along with awake records in children above 5 years of age. Data from all 100 consecutive patients referred for VEEG between January 2021 and January 2022 were noted in an Excel sheet (Microsoft Corp., Redmond, WA, USA) and analysed for predefined variables. All EEG examinations were done using a Nicolet One (Nicolet One V32 amplifier, Natus Neurology, Pleasanton, CA, USA). A 16-channel EEG recording was conducted, using the 10–20 International System of electrode placement with bipolar and referential montages.
1. Statistical analysis
The collected data were recorded in an Excel spreadsheet (Microsoft, Redmond, WA, USA). The statistical analysis was performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). For the descriptive data (e.g., participants’ demographic profile and comorbidities), mean and standard deviation (SD) values were used, and categorical variables were compared using the chi-square or Fisher exact test. The Student t-test was used for parametric continuous variables, the Wilcoxon signed rank test for nonparametric continuous variables, and differences with a P value of 0.05 or lower were considered significant.
2. Ethical statement
The study was approved by Command Hospital, Chandimandir Institute Ethical Committee (letter number 02/27/CHWC/2022). Written informed consent was waived due to retrospective nature of the study.
Results
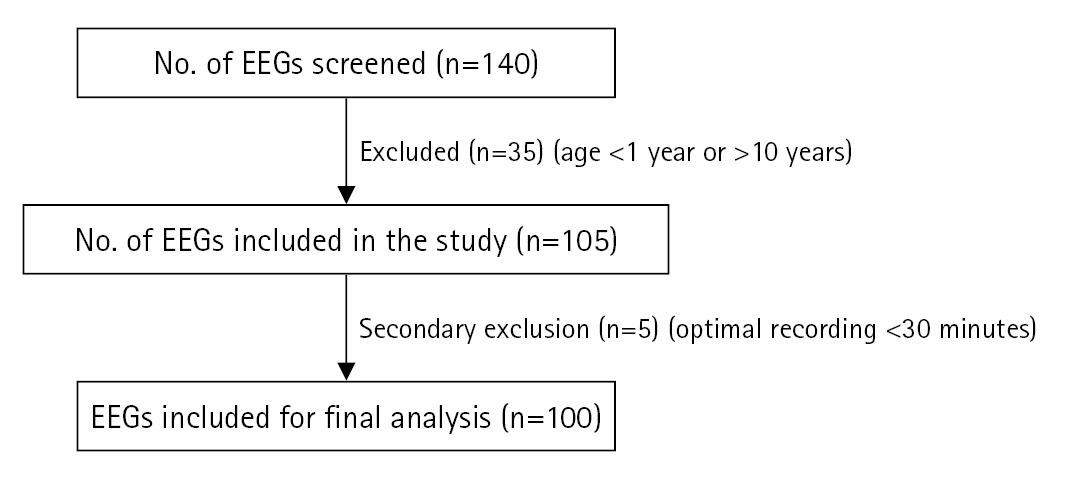
During the study period from January 2021 to January 2022, 140 short-term VEEGs were captured. The EEGs were screened for the inclusion criteria. Those belonging to children aged 1 to 10 years were selected. If the duration of artefact-free recordings was less than 30 minutes, then the EEG was excluded from the analysis (Fig. 1). Finally, 100 EEG recordings were analysed as part of this retrospective study.
Most of the patients (66/100) were boys, and their mean±SD age was 5.4±2.1 years. The indications for requesting an EEG study were varied. The most common disorder for which a clinician requested an EEG examination was epilepsy (72/100). This category was defined as including structural as well as genetic types of epilepsy, epileptic encephalopathy, and evaluations of the first episode of unprovoked seizures. Other diagnoses included autoimmune encephalitis, tuberous sclerosis, paroxysmal non-epileptic episodes, and autistic spectrum disorder (Table 1). Epileptiform abnormalities were found in 58 of these 100 EEG records.
EEGs were recorded either in a sleeping state alone (66/100) or awake as well as asleep (34/100). The two groups were compared. Fifty-three percent of the asleep records showed epileptiform discharges. In contrast, when EEG was captured both in sleeping and awake states, epileptiform discharges were found in 67.6% of records (Table 2). The proportion of EEG examinations with generalised interictal epileptiform discharges was higher in sleeping records (19/35), while a combination of asleep and awake EEG captured focal abnormalities in 13 of 23 patients.
The number of ASMs that these children were taking ranged from 0 to 4. Most children received two ASMs (52/100). The fewest children were observed in the group receiving four ASMs (3/100). The children taking two or more ASMs had a higher proportion of abnormal EEG recordings (P<0.0001) (Table 3).
The proportion of children with abnormal EEGs who were receiving none, one, or two ASMs, was 12.5%, 58%, and 62%, respectively. The proportion of EEG examinations with focal interictal epileptiform discharges was higher in children taking two or fewer ASMs, while those taking three or four ASMs had a higher proportion of generalised interictal epileptiform discharges (P<0.0001).
Discussion
VEEG is a commonly used tool for the diagnosis, evaluation, and classification of childhood epilepsy, to differentiate seizures from seizure mimickers, and for pre-surgical evaluations prior to epilepsy surgery. Our study investigated the role of short-term VEEG in the identification of the nature of abnormal electrical/electro-clinical events and also assessed the utility of VEEG in confirmation/classification of the referring diagnosis.
In our study, we observed a relatively high male-to-female ratio, with the most common reason for referral for VEEG being clinical suspicion of epilepsy, followed by neurodevelopmental disorders, autoimmune disorders, and paroxysmal non-epileptic events (PNEEs). A study by Nordli [6] showed that the main reason for performing VEEG in children was to clarify the nature of paroxysmal events and identify the type of epilepsy syndrome. Similarly, other studies also highlighted the importance of the same reason for using VEEG in children [7,8]. However, in our study, we also found that many of the VEEG examinations were performed in children with neurodevelopmental disorders. This has been a recent trend among pediatric neurologists, and it helps in the early identification of any epileptic activity in children suffering from neurodevelopmental disorders, as well as in identifying whether any of the symptoms in these children could be attributed to an underlying epileptic process. In many studies, pseudo-seizures or PNEEs have also been a common indication for ordering VEEG, accounting for almost 20% of the cases [9]. However, in our study, this was observed in only 4% of cases. The low proportion of referrals for VEEG in patients with suspected PNEEs could be because any epileptiform activity can be easily picked up on routine EEGs, not necessitating a VEEG in all such cases.
We observed that the yield of EEG abnormalities was higher in children in whom both asleep and awake VEEG recordings were obtained than in those with only asleep VEEG recordings, although the difference was not statistically significant. In a study by Delil et al. [10], it was seen that there was a greater likelihood of abnormal awake EEG in patients with generalised epilepsy, especially in cases with a presumed genetic origin. In children, due to various logistic issues, many clinicians have asleep EEG done to detect any epileptiform activity. However, based on these findings, one should make all possible efforts to have both awake and asleep EEG performed in a child with suspected epileptiform activity. The absence of statistical significance could have been due to the small size of our study. It is highly likely that we would obtain statistically significant results if similar studies are replicated with a larger sample.
Another observation was that children who were taking two or more ASMs had a higher incidence of abnormal discharges on short-term VEEG recordings. ASMs affect the origin and propagation of seizures in diverse ways and with different potencies [11]. They suppress seizure activity through two main mechanisms: (1) diffusely inhibiting excitatory synaptic transmission and (2) selectively affecting regions critical for seizures (e.g., the hippocampal formation). A higher number of ASMs indicates that children’s brains have already entered or are entering a state of drug resistance. In such children, the usage of a higher number of ASMs does not significantly reduce epileptiform discharges. Hence, this could be a plausible explanation for the higher incidence of abnormal VEEGs in children taking two or more ASMs. Guida et al. [12] studied the effect of ASMs on refractory focal epilepsy, and opined that in patients with refractory focal epilepsy taking multiple ASMs, a significant reduction in epileptiform discharges was not observed, and frequent repetitive EEGs may not significantly change the management of epilepsy.
A strength of our study is that it involves a diverse set of study subjects from various regions of northern India, ensuring a heterogeneous population for the study. Short-term VEEG examinations help to distinguish between epileptic and non-epileptic events. Therefore, in resource-limited settings, long-term VEEG recordings can be ordered only in cases where short-term EEG examinations do not yield appropriate results.
Our study is not without limitations. Firstly, the sample size was relatively small. Secondly, repeat long-term VEEG examinations were not performed for the 42% of children who did not have any abnormal EEG findings on short-term VEEG. No comparison was made between long-term VEEG and short-term VEEG recordings or between VEEG and non-VEEG recordings, as all our subjects underwent short-term VEEG.
In conclusion, short-term EEG can have a good diagnostic yield and can help in identifying epileptiform discharges, especially in resource-limited settings. However, if normal results are obtained from short-term recordings, long-term VEEG should be conducted not to miss any abnormal electrical activity. In children taking two or more ASMs, the EEG examinations are more likely to show epileptiform discharges. Wherever possible, both asleep and awake EEG with activation procedures should be done to improve the yield of the EEG examinations. A better selection of patients for routine EEG, through an assessment of clinical history and comorbidities, is warranted to increase its diagnostic yield.
Notes
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: RS. Data curation: RS and SS. Formal analysis: GK and AU. Methodology: RS. Visualization: SS. Writing-review & editing: RS, SS, and GK.
Acknowledgements
The authors acknowledge the contribution of all the staff of the Neurology Division.