 |
 |
- Search
| Ann Child Neurol > Volume 29(3); 2021 > Article |
|
Abstract
Purpose
Methods
Results
Supplementary materials
Supplementary Fig. 1.
Conflicts of interest
Jeehun Lee is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Notes
Author contribution
Conceptualization: JL and JL. Data curation: JYS and JL. Formal analysis: JYS. Methodology: JIL, HJS, JL, and JL. Project administration: JL and JL. Visualization: JYS. Writing-original draft: JYS. Writing-review & editing: JL and JL.
Acknowledgments
Fig. 1.
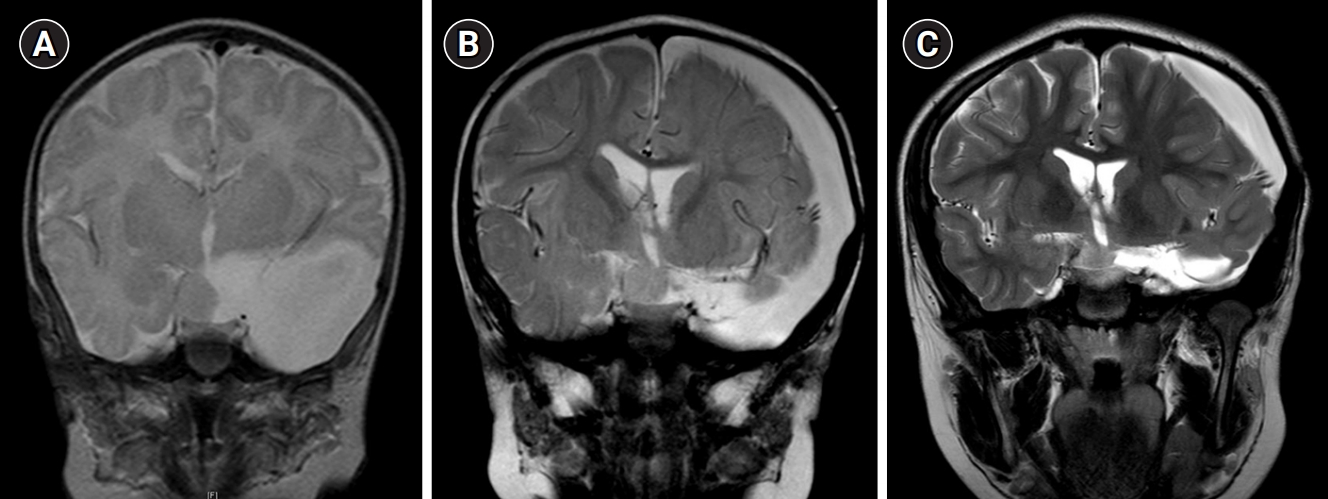
Fig. 2.
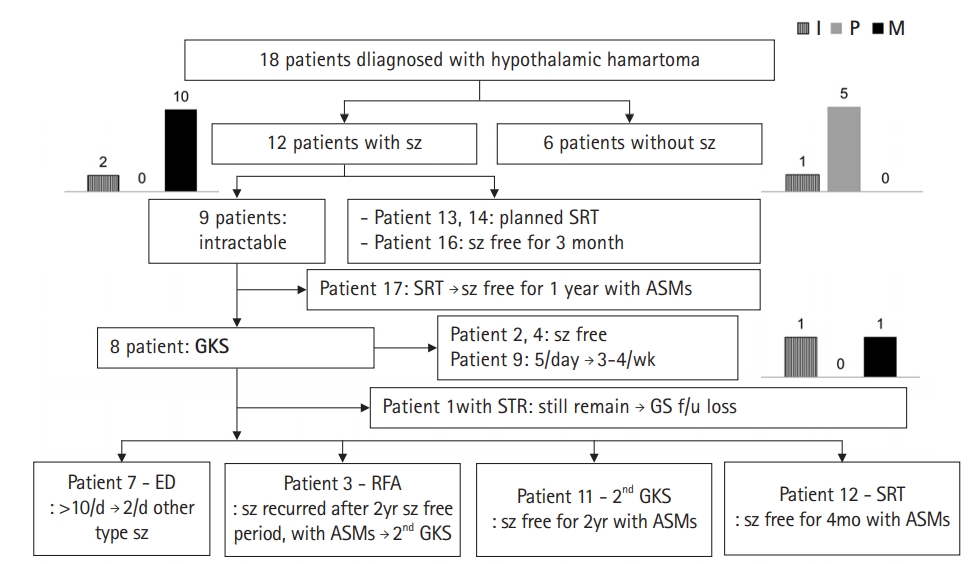
Fig. 3.
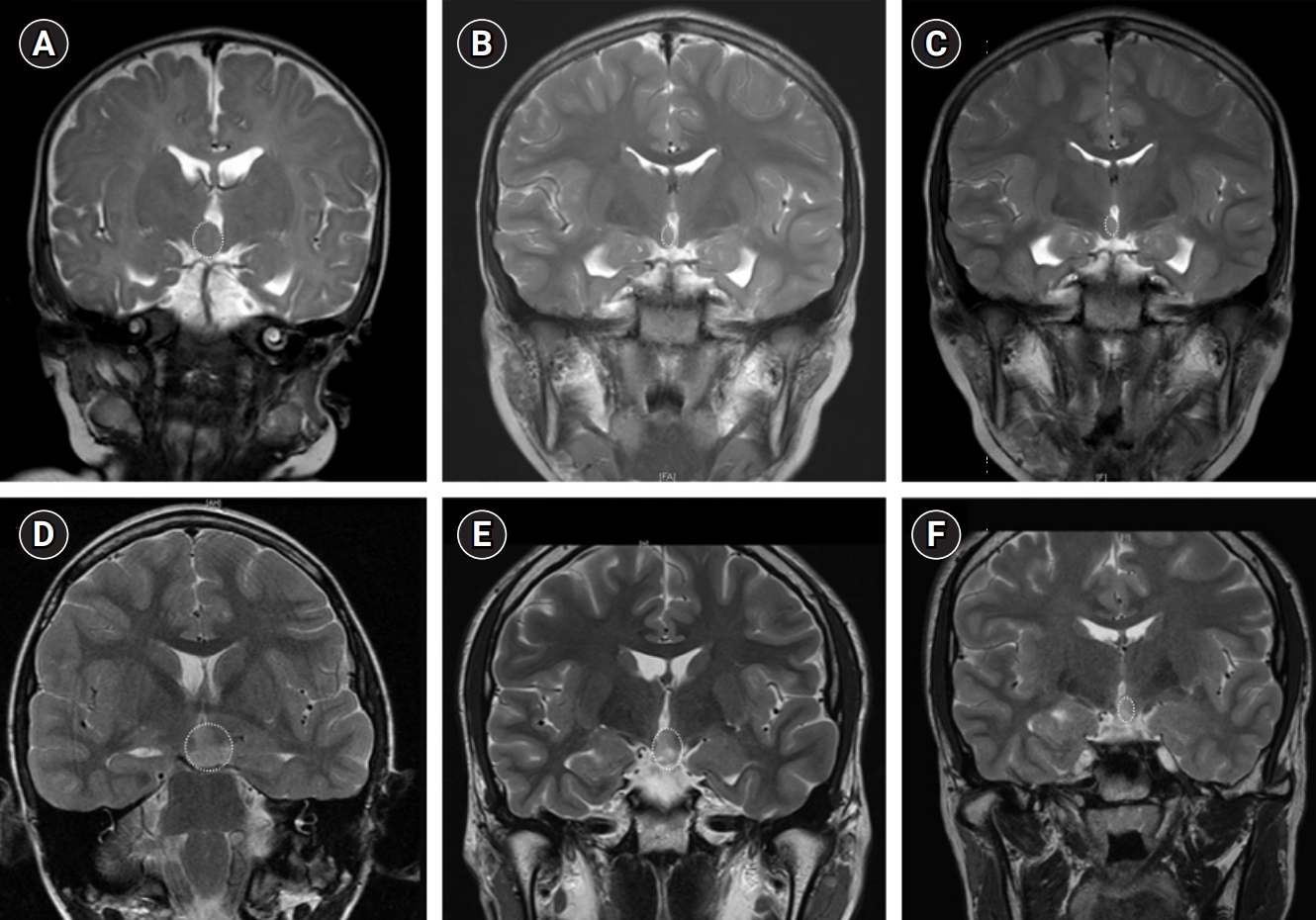
Table 1.
| Case | Sex | Age at diagnosis | FU (yr) |
Presenting symptom (onset age) |
Response to ASM | HH size (long axis, cm) | HH typea | Neuropsychological disorder | |
|---|---|---|---|---|---|---|---|---|---|
| Seizure | PP | ||||||||
| 1 | F | 8 mo | 18.6 | GS (8 mo) | PP (8 mo) | None | 2 | M | None |
| FSIA,GTC (8 mo) | |||||||||
| 2 | F | 6 yr | 11.3 | FSIA (6 yr) | PP (6 yr) | None | 2.7 | M | Mild ID: FSIQ 75 |
| 3 | M | 3 yr | 11.8 | GS (3 yr) | PP (6.5 yr) | None | 1.8 | M | Mild ID: FSIQ 81 |
| FSIA,GTC (6 yr) | ADHD | ||||||||
| 4 | M | 3.5 yr | 5 | GS (1 mo) | - | Partial response | 1.8 | M | None |
| 5 | F | 2.5 yr | 8 | - | PP (2.5 yr) | NA | 2 | P | None |
| 6 | M | 10.5 yr | 6.7 | - | PP (10.5 yr) | NA | 1 | P | None |
| 7 | M | 5 yr | 9.9 | GS (3.8 yr) | PP (9 yr) | None | 1 | M | Moderate ID: FSIQ 46 |
| FSIA, GT (8 yr) | |||||||||
| 8 | M | 1 mo | 10.3 | - | - | NA | 2 | P | None |
| 9 | F | 8 yr | 12.2 | GS (1 mo) | - | None | 1.2 | I | Mild ID: FSIQ 75 |
| FSIA (13 yr) | Paranoid PD | ||||||||
| 10 | F | 2.7 yr | 8.2 | - | PP (2.7 yr) | NA | 1.6 | P | None |
| 11 | M | 1 yr | 11.6 | GS (1 yr) | - | None | 1.1 | I | None |
| 12 | F | 4 yr | 2.5 | GS (4 yr) | PP (7.5 yr) | Partial response | 0.9 | M | None |
| 13 | F | 8 mo | 0.4 | GS (3 mo) | - | None | 2 | M | None |
| 14 | M | 26 mo | 1 | GS (26 mo) | PP (3.3 yr) | None | 1.2 | M | None |
| 15 | F | 3.4 yr | 1.5 | - | PP (4 yr) | NA | 1.3 | P | None |
| 16 | M | 10 yr | 0.3 | GS (7 yr) | - | Partial response | 0.9 | M | None |
| 17 | M | 2 yr | 1.9 | GS (8 mo) | - | Partial response | 0.8 | M | None |
| 18 | M | 5 yr | 0.3 | - | PP (5 yr) | NA | 0.8 | I | None |
FU, follow-up; PP, precocious puberty; ASM, anti-seizure medication; HH, hypothalamic hamartoma; GS, gelastic seizure; FSIA, focal seizure with impaired awareness; GTC, generalized tonic clonic seizure; M, mixed hypothalamic; ID, intellectual disability; P, parahypothalamic; FSIQ, full scale intelligence quotient; ADHD, attention deficit hyperactivity disorder; NA, not available; I, intrahypothalamic; PD, personality disorder.
a HH types according to Kameyama et al. [16].
Table 2.
| Case |
HH on brain MRI |
Operation (age, yr) | FU after last operation (yr) | Radiation dose (Gy) |
Size change of HH after operation (maximum diameter, mm) |
Seizure outcomes after operation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sizea (mm) | Typeb | Type of seizure: reduction of frequency |
No. of ASMs |
Engel’s/ILAE classificationc | ||||||
| Pre | Post | |||||||||
| 1 | 20 | M | STR (1.5) | - | - | -d | GS: <10% | 1 | 1 | ID/Class 4 |
| GKS (13.2) | 6 | 15 | No change | FSIA, GTC: seizure-free | 1 | 1 | ||||
| 2 | 27 | M | GKS (7) | 11 | 12 | No change | FSIA: seizure-free | 1 | None | IA/Class 1 |
| 3 | 18 | M | GKS (6.5) | - | 17 | Minimal decrease (17→after 4 years: 10) | GS: >50% | 1 | None | ID/Class 4 |
| RFA (15.1) | - | - | >50% decrease (7) | FSIA, GTC: seizure-free | 2 | 1 | ||||
| GKS (17.1) | 1 | 18 | No change | 1 | 2 | |||||
| 4 | 18 | M | GKS (4.8) | 4 | 17 | Minimal decrease (16) | GS: seizure-free | 3 | None | IA/Class 1 |
| 7 | 10 | M | GKS (6) | - | 17 | No change | GS: >50% | 4 | 4 | IVC/Class 6 |
| ED (7.4) | 8 | >50% decrease (10) | FSIA, GT: newly appeared | 4 | 1→5 | |||||
| 9 | 12 | I | GKS (15.5) | 3 | 17 | Minimal decrease (11.5→after 4 years: 8) | GS: <10% | 3 | 2 | IA/Class 1 |
| FSIA: seizure-free | ||||||||||
| 11 | 11 | I | GKS (6.9) | - | 18 | Minimal decrease (9) | GTC: seizure-free | 2 | 3 | IA/Class 1 |
| GKS (9.7) | 2 | 17 | Minimal decrease (7) | GS: seizure-free | 3 | 2 | ||||
| 12 | 9 | M | GKS (5.1) | - | 18 | No change | GS: <50%→free for 4 months | 2 | 1 | IB/Class 4 |
| SRT (7.1) | 0.3 | - | - | 1 | 1 | |||||
| 17 | 9 | M | SRT (3.2) | 2 | - | >50% decrease (not visible) | GS: recur after 6 months | 2 | None | IB/Class 4 |
| FSIA: seizure-free | ||||||||||
HH, hypothalamic hamartoma; MRI, magnetic resonance imaging; FU, follow-up; ASM, anti-seizure medication; ILAE, International League Against Epilepsy; M, mixed hypothalamic; STR, subtotal resection; GKS, gamma-knife radiosurgery; GS, gelastic seizure; FSIA, focal seizure with impaired awareness; GTC, generalized tonic clonic seizure; RFA, radiofrequency ablation; ED, endoscopic disconnection; GT, generalized tonic seizure; I, intrahypothalamic; SRT, stereotactic radiofrequency thermocoagulation.
b HH types according to Kameyama et al. [16];
Table 3.
| Study | Year | Treatment | Follow-up (median) | No. of patients | Age at operation (median) | Size of Hha (median) | Seizure outcomesb | Side effects (no. or % of cases) |
|---|---|---|---|---|---|---|---|---|
| Curry et al. [8] (Texas) | 2011-2018 | LiTT (MRI-guided) | 12 mo (12) | 71 | 5 mo-20 yr | (4-30 mm) | Free from GS: 93% | DI (1) |
| Free from seizure and medication: 12% | Severe deficit of short-term memory (1-history of right temporal lobectomy) | |||||||
| Episodic hyponatremia (3) | ||||||||
| Gadgil et al. [31] (Texas) | 2011-2017 | LiTT (MRI-guided) | 1.2 yr (0.6-6.3) | 58 | 5.5 yr (0.4-20.9) | 0.52 mL (0.06-14.49) | Engel I+II: 81.1% (69.0%+12.1%) | Disorders of sodium metabolism (6.9%) |
| Memory disturbance (8.6%) | ||||||||
| Unfavorable catheter positioning (1.7%) | ||||||||
| Ferrand et al. [32] (Rothschild) | 1998-2017 | ED | 37 mo (13-77) | 112 | 7.6 yr (48-133 mo) | NA | Engel I+II: 77.6% (57.1 %+20.5 %) | DI: transient (2), permanent (1) |
| CN III palsy: transient (9), permanent (3) | ||||||||
| Memory deficit (4), motor deficit (5), | ||||||||
| SUDEP (1), weight gain (5) | ||||||||
| Shirozu et al. [12] (Niigata) | 1997-2013 | RFA | 3 yr (1-17) | 100 | 10.0 yr (1-50) | 15 mm (5-80) | Free from GS: 86.0% | Delayed precocious puberty (9.0%) |
| Free from other types of seizures: 78.9% | Pituitary dysfunction (2.0%) | |||||||
| Weight gain (7.0%) | ||||||||
| Regis et al. [33] (Marseille) | 1999-2007 | GKS | 71 mo (36-153) | 48 | 16.5 yr (3-50) | 9 mm (4-30) | Engel I+II: 65% (47.5%+17.5%) | No permanent neurologic side effects |
| Transient poikilothermia (6.2%) | ||||||||
| Transient seizure increase (16.6%) | ||||||||
| Abla et al. [34] (Barrow) | 2003-2010 | GKS | Mean: 43 mo (18-81) | 10 | Mean: 15.1 yr (5.7-29.3) | Mean: 0.2 mL (0.14-0.28) | Free from seizures: 60% | Short-term memory loss (3) |
| 50%-90% reduction: 10% | Poikilothermia (1) | |||||||
| 50% reduction: 20% | Increased depression (1) | |||||||
| Weight gain/increased appetite (2) | ||||||||
| Anxiety (1) | ||||||||
| Mathieu et al. [35] (Sherbrooke) | Report from 2010 | GKS | 36 mo (6-56) | 9 | 23 yr (12-57) | 0.6 mL (0.3-1.0) | Engel I: 4 (44.4%) | No side effects after the procedure |
| Engel II: 1 (11.1%) |
HH, hypothalamic hamartoma; LiTT, laser interstitial thermal therapy; MRI, magnetic resonance imaging; GS, gelastic seizure; DI, diabetes insipidus; ED, endoscopic disconnection; NA, not available; CN, cranial nerve; SUDEP, sudden unexpected death in epilepsy; RFA, radiofrequency ablation; GKS, gamma knife radiosurgery.
References
- TOOLS







